FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
1% Teat Dip Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
FIRMAGON is a GnRH receptor antagonist indicated for treatment of patients with advanced prostate cancer.
History
There is currently no drug history available for this drug.
Other Information
FIRMAGON is a sterile lyophilized powder for injection containing degarelix (as the acetate) and mannitol. Degarelix is a synthetic linear decapeptide amide containing seven unnatural amino acids, five of which are D-amino acids. The acetate salt of degarelix is a white to off-white amorphous powder of low density as obtained after lyophilization.
The chemical name of degarelix is D-Alaninamide, N-acetyl-3-(2-naphthalenyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-4-[[[(4S)-hexahydro-2,6-dioxo-4-pyrimidinyl]carbonyl]amino]-L phenylalanyl-4-[(aminocarbonyl)amino]-D-phenylalanyl-L leucyl-N6–(1-methylethyl)-L-lysyl-L-prolyl. It has an empirical formula of C82H103N18O16Cl and a molecular weight of 1632.3 Da.
Degarelix has the following structural formula:
FIRMAGON delivers degarelix acetate, equivalent to 120 mg of degarelix for the starting dose, and 80 mg of degarelix for the maintenance dose. The 80 mg vial contains 200 mg mannitol and the 120 mg vial contains 150 mg mannitol.
Sources
1% Teat Dip Manufacturers
-
Agri-services Llc
![1% Teat Dip (Iodine) Solution [Agri-services Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
1% Teat Dip | Ferring Pharmaceuticals Inc.
![1% Teat Dip (Iodine) Solution [Agri-services Llc] 1% Teat Dip (Iodine) Solution [Agri-services Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
FIRMAGON is for subcutaneous administration only.
Dosing information:
Starting dose Maintenance dose – Administration every 28 days 240 mg given as two subcutaneous injections of 120 mg at a concentration of 40 mg/mL 80 mg given as one subcutaneous injection at a concentration of 20 mg/mL
The first maintenance dose should be given 28 days after the starting dose.
FIRMAGON is administered as a subcutaneous injection in the abdominal region. As with other drugs administered by subcutaneous injection, the injection site should vary periodically. Injections should be given in areas of the abdomen that will not be exposed to pressure, e.g., not close to waistband or belt nor close to the ribs.
FIRMAGON is supplied as a powder to be reconstituted with Sterile Water for Injection, USP (WFI).
The instruction for reconstitution needs to be carefully followed. Administration of other concentrations is not recommended. See Instructions for Proper Use.
Instructions for Reconstitution
The following information is intended for healthcare professionals only:
NOTE:
Reconstituted drug must be administered within one hour after addition of Sterile Water for Injection, USP Do not shake the vials Follow aseptic techniqueFIRMAGON 240 mg – Starting Dose
The pack contains 2 sets of FIRMAGON 120 mg vial, Sterile Water for Injection, USP syringe, vial adapter, and administration needle. For each subcutaneous injection, the drug product must be prepared using the following instructions.
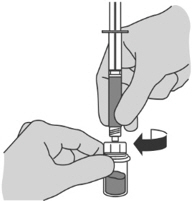
1. Uncap vial and wipe the vial rubber stopper with an alcohol pad. Remove the cover from the vial adapter package. Attach the vial adapter to the vial by pressing the adapter down until the spike pushes through the rubber stopper and the adapter snaps in place (see Figure A). 2. Assembly of the syringe is required. Prepare the prefilled syringe by attaching the plunger. 3. Remove the cap of the prefilled syringe. Attach the syringe to the vial by screwing it on to the adapter. Transfer all Sterile Water for Injection, USP to the vial (see Figure B). 4. With the syringe still attached to the adapter, swirl gently until the liquid looks clear and free of powder or particles (see Figure C). If the powder adheres to the side of the vial above the liquid surface, the vial can be tilted slightly. Avoid shaking to prevent foam formation.
A ring of small air bubbles on the surface of the liquid is acceptable. The reconstitution procedure may take, in some cases, up to 15 minutes, but usually takes a few minutes.
5. Turn the vial upside down and draw up to the 3 mL mark on the syringe for injection (see Figure D).
Always make sure to withdraw the precise volume and expel any air bubbles. 6. Detach the syringe from the vial adapter and attach the administration needle to the syringe.
7. Immediately after reconstitution, inject 3 mL of FIRMAGON 120 mg slowly.
Perform a deep subcutaneous injection.
To do so: pinch the skin of the abdomen, elevate the subcutaneous tissue and insert the needle at a 45 degree angle (see Figure E).
Do not inject into a vein or muscle. Gently pull back the plunger to check if blood is aspirated. If blood appears in the syringe, the product can no longer be used. Discontinue the injection and discard the syringe and the needle (reconstitute a new dose for the patient).
No injections should be given in areas where the patient will be exposed to pressure, e.g., around the belt or waistband or close to the ribs. 8. Repeat the reconstitution procedure for the second 120 mg vial to complete the 240 mg starting dose and choose a different injection site for the second dose.FIRMAGON 80 mg – Maintenance Dose
The pack contains 1 set of FIRMAGON 80 mg vial, Sterile Water for Injection, USP syringe, vial adapter, and administration needle. For the subcutaneous injection, the drug product must be prepared using the following instructions.
1. Uncap vial and wipe the vial rubber stopper with an alcohol pad. Remove the cover from the vial adapter package. Attach the adapter to the powder vial by pressing the adapter down until the spike pushes through the rubber stopper and the adapter snaps in place (see Figure A). 2. Assembly of the syringe is required. Prepare the prefilled syringe by attaching the plunger. 3. Remove the cap of the prefilled syringe. Attach the syringe to the vial by screwing it on to the adapter. Transfer all Sterile Water for Injection, USP to the vial (see Figure B). 4. With the syringe still attached to the adapter, swirl gently until the liquid looks clear and free of powder or particles (see Figure C). If the powder adheres to the side of the vial above the liquid surface, the vial can be tilted slightly. Avoid shaking to prevent foam formation.
A ring of small air bubbles on the surface of the liquid is acceptable. The reconstitution procedure may take, in some cases, up to 15 minutes, but usually takes a few minutes. 5. Turn the vial upside down and draw up to the 4 mL mark on the syringe for injection (see Figure D).
Always make sure to withdraw the precise volume and expel any air bubbles. 6. Detach the syringe from the vial adapter and attach administration needle to the syringe. 7. Immediately after reconstitution, inject 4 mL of FIRMAGON 80 mg slowly.
Perform a deep subcutaneous injection.
To do so: pinch the skin of the abdomen, elevate the subcutaneous tissue and insert the needle at a 45 degree angle (see Figure E).
Do not inject into a vein or muscle. Gently pull back the plunger to check if blood is aspirated. If blood appears in the syringe, the product can no longer be used. Discontinue the injection and discard the syringe and the needle (reconstitute a new dose for the patient).
No injections should be given in areas where the patient will be exposed to pressure, e.g., around the belt or waistband or close to the ribs.
Login To Your Free Account