FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Acyclovir Sodium Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Acyclovir Sodium Injection is intended for intravenous infusion only, and should not be administered topically, intramuscularly, orally, subcutaneously, or in the eye. Intravenous infusions must be given over a period of at least 1 hour to reduce the risk of renal tubular damage (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Renal failure, in some cases resulting in death, has been observed with acyclovir therapy (see ADVERSE REACTIONS, Observed During Clinical Practice and OVERDOSAGE). Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), which has resulted in death, has occurred in immunocompromised patients receiving acyclovir therapy.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Acyclovir Sodium Injection is indicated for the treatment of initial and recurrent mucosal and cutaneous herpes simplex (HSV-1 and HSV-2) in immunocompromised patients.
Acyclovir Sodium Injection is indicated for the treatment of severe initial clinical episodes of herpes genitalis in immuno-competent patients.
Acyclovir Sodium Injection is indicated for the treatment of herpes simplex encephalitis.
Acyclovir Sodium Injection is indicated for the treatment of neonatal herpes infections.
Acyclovir Sodium Injection is indicated for the treatment of varicella-zoster (shingles) infections in immunocompromised patients.
History
There is currently no drug history available for this drug.
Other Information
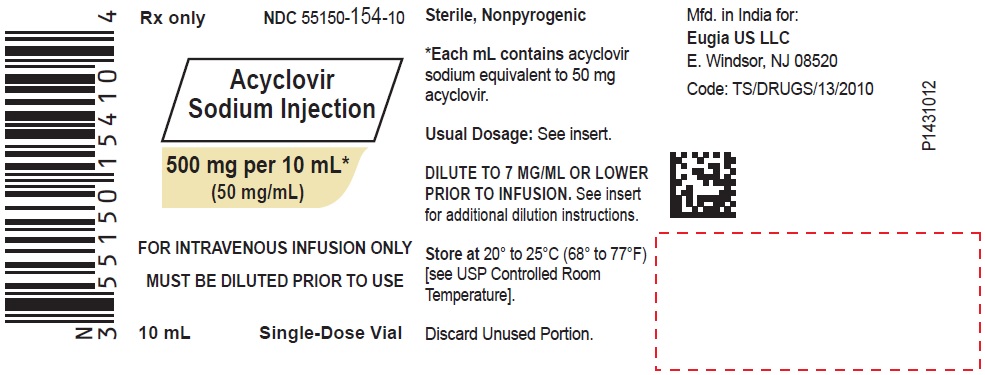
Acyclovir Sodium Injection is a synthetic nucleoside analog, active against herpes viruses. It is a sterile, aqueous solution for intravenous infusion, containing 50 mg acyclovir per mL in Water for Injection, USP. The concentration is equivalent to 54.9 mg of acyclovir sodium per mL in Water for Injection, USP. The sodium content is approximately 5.1 mg/mL. The pH range of the solution is 10.85 to 11.50. Further dilution of Acyclovir Sodium Injection in an appropriate intravenous solution must be performed before infusion (see DOSAGE AND ADMINISTRATION, Administration).
The chemical name of acyclovir sodium is 9-[(2-Hydroxyethoxy)methyl] guanine, and has the following structural formula:

Acyclovir USP is a white to off-white, crystalline powder. Acyclovir sodium is the sodium salt of acyclovir, which is formed in situ, with the molecular formula C8H10N5NaO3 and a molecular weight of 247.19. The maximum solubility in water at 25°C exceeds 100 mg/mL. At physiologic pH, acyclovir sodium exists as the unionized form with a molecular weight of 225 and a maximum solubility in water at 37°C of 2.5 mg/mL. The pka’s of acyclovir are 2.27 and 9.25.
Sources
Acyclovir Sodium Manufacturers
-
Auromedics Pharma Llc
![Acyclovir Sodium Injection, Solution [Auromedics Pharma Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Acyclovir Sodium | Auromedics Pharma Llc
![Acyclovir Sodium Injection, Solution [Auromedics Pharma Llc] Acyclovir Sodium Injection, Solution [Auromedics Pharma Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
CAUTION - RAPID OR BOLUS INTRAVENOUS INJECTION MUST BE AVOIDED (see WARNINGS and PRECAUTIONS).
Dosage
INTRAMUSCULAR OR SUBCUTANEOUS INJECTION MUST BE AVOIDED (see WARNINGS).
Therapy should be initiated as early as possible following onset of signs and symptoms of herpes infections.
A maximum dose equivalent to 20 mg/kg every 8 hours should not be exceeded for any patient.HERPES SIMPLEX INFECTIONS
MUCOSAL AND CUTANEOUS HERPES SIMPLEX (HSV-1 AND HSV-2) INFECTIONS IN IMMUNOCOMPROMISED PATIENTS
Adults and Adolescents (12 years of age and older)
5 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days.
Pediatrics (Under 12 years of age)
10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days.
SEVERE INITIAL CLINICAL EPISODES OF HERPES GENITALIS
Adults and Adolescents (12 years of age and older)
5 mg/kg infused at a constant rate over 1 hour, every 8 hours for 5 days.
HERPES SIMPLEX ENCEPHALITIS
Adults and Adolescents (12 years of age and older)
10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 10 days.
Pediatrics (3 months to 12 years of age)
20 mg/kg infused at a constant rate over 1 hour, every 8 hours for 10 days.
Neonatal Herpes Simplex Virus Infections (Birth to 3 months)
10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 10 days. In neonatal herpes simplex infections, doses of 15 mg/kg or 20 mg/kg (infused at a constant rate over 1 hour every 8 hours) have been used; the safety and efficacy of these doses are not known.
VARICELLA-ZOSTER INFECTIONS
ZOSTER IN IMMUNOCOMPROMISED PATIENTS
Adults and Adolescents (12 years of age and older)
10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days.
Pediatrics (Under 12 years of age)
20 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days.
Obese Patients
Obese patients should be dosed at the recommended adult dose using Ideal Body Weight.
PATIENTS WITH ACUTE OR CHRONIC RENAL IMPAIRMENT
Refer to DOSAGE AND ADMINISTRATION section for recommended doses, and adjust the dosing interval as indicated in Table 5.
Table 5: Dosage Adjustments for Patients with Renal Impairment Creatinine Clearance
(mL/min/1.73 m2) Percent of
Recommended Dose Dosing Interval
(hours) >50
100%
8
25 to 50
100%
12
10 to 25
100%
24
0 to 10
50%
24
HemodialysisFor patients who require dialysis, the mean plasma half-life of acyclovir during hemodialysis is approximately 5 hours. This results in a 60% decrease in plasma concentrations following a six-hour dialysis period. Therefore, the patient’s dosing schedule should be adjusted so that an additional dose is administered after each dialysis.
Peritoneal DialysisNo supplemental dose appears to be necessary after adjustment of the dosing interval.
AdministrationThe calculated dose should be further diluted in an appropriate intravenous solution at a volume selected for administration during each 1 hour infusion. Infusion concentrations of approximately 7 mg/mL or lower are recommended. In clinical studies, the average 70 kg adult received between 60 and 150 mL of fluid per dose. Higher concentrations (e.g., 10 mg/mL) may produce phlebitis or inflammation at the injection site upon inadvertent extravasation. Standard, commercially available electrolyte and glucose solutions are suitable for intravenous administration; biologic or colloidal fluids (e.g., blood products, protein solutions, etc.) are not recommended.
Once diluted for administration, each dose should be used within 24 hours.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. -
Auromedics Pharma Llc
![Acyclovir Sodium Injection, Solution [Auromedics Pharma Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Acyclovir Sodium | Auromedics Pharma Llc
![Acyclovir Sodium Injection, Solution [Auromedics Pharma Llc] Acyclovir Sodium Injection, Solution [Auromedics Pharma Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
CAUTION - RAPID OR BOLUS INTRAVENOUS INJECTION MUST BE AVOIDED (see WARNINGS and PRECAUTIONS).
Dosage
INTRAMUSCULAR OR SUBCUTANEOUS INJECTION MUST BE AVOIDED (see WARNINGS).
Therapy should be initiated as early as possible following onset of signs and symptoms of herpes infections.
A maximum dose equivalent to 20 mg/kg every 8 hours should not be exceeded for any patient.HERPES SIMPLEX INFECTIONS
MUCOSAL AND CUTANEOUS HERPES SIMPLEX (HSV-1 AND HSV-2) INFECTIONS IN IMMUNOCOMPROMISED PATIENTS
Adults and Adolescents (12 years of age and older)
5 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days.
Pediatrics (Under 12 years of age)
10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days.
SEVERE INITIAL CLINICAL EPISODES OF HERPES GENITALIS
Adults and Adolescents (12 years of age and older)
5 mg/kg infused at a constant rate over 1 hour, every 8 hours for 5 days.
HERPES SIMPLEX ENCEPHALITIS
Adults and Adolescents (12 years of age and older)
10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 10 days.
Pediatrics (3 months to 12 years of age)
20 mg/kg infused at a constant rate over 1 hour, every 8 hours for 10 days.
Neonatal Herpes Simplex Virus Infections (Birth to 3 months)
10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 10 days. In neonatal herpes simplex infections, doses of 15 mg/kg or 20 mg/kg (infused at a constant rate over 1 hour every 8 hours) have been used; the safety and efficacy of these doses are not known.
VARICELLA-ZOSTER INFECTIONS
ZOSTER IN IMMUNOCOMPROMISED PATIENTS
Adults and Adolescents (12 years of age and older)
10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days.
Pediatrics (Under 12 years of age)
20 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days.
Obese Patients
Obese patients should be dosed at the recommended adult dose using Ideal Body Weight.
PATIENTS WITH ACUTE OR CHRONIC RENAL IMPAIRMENT
Refer to DOSAGE AND ADMINISTRATION section for recommended doses, and adjust the dosing interval as indicated in Table 5.
Table 5: Dosage Adjustments for Patients with Renal Impairment Creatinine Clearance
(mL/min/1.73 m2) Percent of
Recommended Dose Dosing Interval
(hours) >50
100%
8
25 to 50
100%
12
10 to 25
100%
24
0 to 10
50%
24
HemodialysisFor patients who require dialysis, the mean plasma half-life of acyclovir during hemodialysis is approximately 5 hours. This results in a 60% decrease in plasma concentrations following a six-hour dialysis period. Therefore, the patient’s dosing schedule should be adjusted so that an additional dose is administered after each dialysis.
Peritoneal DialysisNo supplemental dose appears to be necessary after adjustment of the dosing interval.
AdministrationThe calculated dose should be further diluted in an appropriate intravenous solution at a volume selected for administration during each 1 hour infusion. Infusion concentrations of approximately 7 mg/mL or lower are recommended. In clinical studies, the average 70 kg adult received between 60 and 150 mL of fluid per dose. Higher concentrations (e.g., 10 mg/mL) may produce phlebitis or inflammation at the injection site upon inadvertent extravasation. Standard, commercially available electrolyte and glucose solutions are suitable for intravenous administration; biologic or colloidal fluids (e.g., blood products, protein solutions, etc.) are not recommended.
Once diluted for administration, each dose should be used within 24 hours.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Login To Your Free Account


![Acyclovir Sodium Injection, Solution [Auromedics Pharma Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d3ab226c-677d-4cb7-85cb-1c2e5012f40d&name=acyclovir-fig1.jpg)