FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Arth Rx Topical Analgesic Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
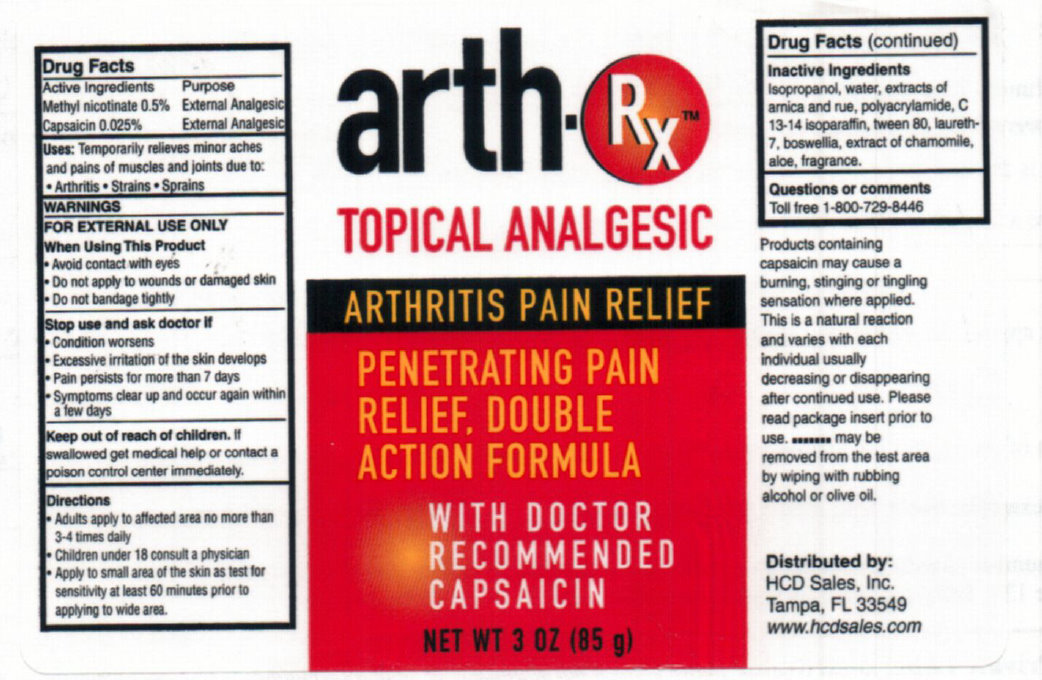
WARNINGS
FOR EXTERNAL USE ONLY
Stop use and ask doctor if
- Condition worsens
- Excessive irritation of the skin develops
- Pain persists for more than 7 days
- Symptoms clear up and occur again within a few days
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
When Using This Product
- Avoid contact with eyes
- Do not apply to wounds or damaged skin
- Do not bandage tightly
History
There is currently no drug history available for this drug.
Other Information
Products containing capsaicin may cause a burning, stinging or tingling sensation where applied. This is a natural reaction and varies with each individual usually decreasing or disappearing after continued use. Please read package insert prior to use. .......... may be removed from the test area by wiping or olive oil.
Distributed by:
HCD sales, Inc,
Tampa, FL 33549
www.hcdsales.com
Sources
Arth Rx Topical Analgesic Manufacturers
-
Hcd Sales
Login To Your Free Account