FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Cinryze Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
CINRYZE is a C1 esterase inhibitor indicated for routine prophylaxis against angioedema attacks in adolescent and adult patients with Hereditary Angioedema (HAE).
History
There is currently no drug history available for this drug.
Other Information
CINRYZE (C1 esterase inhibitor [human]) (Freeze-Dried powder for Reconstitution) is a sterile, stable, lyophilized preparation of C1 esterase inhibitor derived from human plasma. CINRYZE is manufactured from human plasma purified by a combination of filtration and chromatographic procedures. The specific activity of CINRYZE is 4.0 – 9.0 units/mg protein. The purity is ≥ 90% human C1 esterase inhibitor. Following reconstitution with 5 mL of Sterile Water for Injection, USP, each vial contains approximately 500 units of functionally active C1 esterase inhibitor, pH 6.6 - 7.4, and an osmolality between 200 – 400 mosmol/kg. One Unit (U) of CINRYZE corresponds to the mean quantity of C1 esterase inhibitor present in 1 mL of normal fresh plasma.
CINRYZE, when reconstituted with 5 mL of Sterile Water for Injection, USP contains the following excipients: 4.1 mg/mL sodium chloride, 21 mg/mL sucrose, 2.6 mg/mL trisodium citrate, 2.0 mg/mL L-Valine, 1.2 mg/mL L-Alanine, and 4.5 mg/mL L-Threonine.
The following manufacturing steps are designed to reduce the risk of viral transmission:
- Screening donors at U.S. licensed blood collection centers to rule out infection with Human Immunodeficiency Virus (HIV-1/HIV-2), Hepatitis B Virus, or Hepatitis C Virus.
- Testing plasma pools by in-process NAT for parvovirus B19 via minipool testing and the limit of B19 in the manufacturing pool is set not to exceed 104 IU of B19 DNA per mL.
- Use of two independent viral reduction steps in the manufacture of CINRYZE: pasteurization (heat treatment at 60°C for 10 hours in solution with stabilizers) and nanofiltration through two sequential 15 nm filters.
These viral reduction steps, along with a step in the manufacturing process, PEG precipitation, have been validated in a series of in vitro experiments for their capacity to inactivate/remove a wide range of viruses of diverse physicochemical characteristics including: Human Immunodeficiency Virus (HIV), Hepatitis A Virus (HAV), and the following model viruses: Bovine Viral Diarrhea Virus (BVDV) as a model virus for HCV, Canine Parvovirus (CPV) as a model virus for Parvovirus B19, Pseudorabies Virus (PRV) as a model virus for large enveloped DNA viruses (e.g. herpes virus). Total mean log10 reductions are shown in Table 4.
| Process step | Log10 Virus Reduction | ||||
| Enveloped viruses | Non-enveloped viruses | ||||
| HIV | BVDV | PRV | HAV | CPV | |
| PEG precipitation | 5.1 ± 0.2 | 4.5 ± 0.3 | 6.0 ± 0.3 | 2.8 ± 0.2 | 4.2 ± 0.2 |
| Pasteurization | > 6.1 ± 0.2 | > 6.7 ± 0.3 | > 6.7 ± 0.2 | 2.8 ± 0.3 | 0.1 ± 0.3 |
| Nanofiltration | > 5.6 ± 0.2 | > 5.5 ± 0.2 | > 6.4 ± 0.3 | > 4.9 ± 0.2 | > 4.5 ± 0.3 |
| Total reduction | > 16.8 | > 16.7 | > 19.1 | > 10.5 | > 8.7 |
Sources
Cinryze Manufacturers
-
Viropharma Biologics
![Cinryze (Human C1-esterase Inhibitor) Injection, Powder, Lyophilized, For Solution [Viropharma Biologics]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Cinryze | Viropharma Biologics
![Cinryze (Human C1-esterase Inhibitor) Injection, Powder, Lyophilized, For Solution [Viropharma Biologics] Cinryze (Human C1-esterase Inhibitor) Injection, Powder, Lyophilized, For Solution [Viropharma Biologics]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
For Intravenous Use Only.
2.1 Routine prophylaxis against HAE Attacks A dose of 1,000 Units CINRYZE can be administered every 3 or 4 days for routine prophylaxis against angioedema attacks in HAE patients. CINRYZE is administered at an injection rate of 1 mL per minute. Table 1 Routine Prophylaxis Dosing Indication Dose Infusion rate Routine prophylaxis against HAE attacks 1,000 Units
Intravenous
every 3 or 4 days 1 mL/min
(10 min) 2.2 Instructions for UseThe procedures below are provided as general guidelines for the reconstitution and administration of CINRYZE. Use either the Mix2Vial® transfer device or a commercially available double-ended needle.
Always work on a clean surface and wash your hands before performing the following procedures.
Reconstitution, product administration, and handling of the administration set and needles must be done with caution. Percutaneous puncture with a needle contaminated with blood can transmit infectious viruses including HIV (AIDS) and hepatitis. Obtain immediate medical attention if injury occurs. Place needles in a sharps container after single use. Discard all equipment, including any reconstituted CINRYZE in an appropriate container.
2.3 Preparation and Handling Protect CINRYZE from light prior to reconstitution. A silicone-free syringe is recommended for reconstitution and administration of CINRYZE. Inspect the reconstituted product for particulate matter prior to administration; do not use if particles are observed or if solution is turbid. The reconstituted solution is colorless to slightly blue. Each vial of CINRYZE is for single use only. Promptly use any vial that has been entered and discard partially used vials in accordance with biohazard procedures. CINRYZE contains no preservative. Do not mix CINRYZE with other materials. Do not use if frozen. Do not use after expiration date.Reconstitution:
Two vials of reconstituted CINRYZE are combined for a single dose. Sterile Water for Injection, USP, is required and not supplied with CINRYZE.
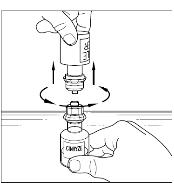
Aseptic technique should be used during the reconstitution procedure. Bring the CINRYZE (powder) and Sterile Water for Injection, USP (diluent) (not supplied) to room temperature if refrigerated. Remove caps from the CINRYZE and diluent vials. Cleanse stoppers with an alcohol wipe or swab, and allow them to dry prior to use. Remove protective covering from the top of the Mix2Vial transfer device package. Do not remove the device from the package. Note: Diluent vial must be accessed prior to the vial of CINRYZE to prevent loss of vacuum. Place diluent on a flat surface and insert the blue end of the device into the diluent vial, pushing down until the spike penetrates through the center of the diluent vial stopper and the device snaps in place (Figure 1). The Mix2Vial transfer device must be positioned completely vertical prior to penetrating the stopper closure. Remove the plastic package and discard it (Figure 2). Take care not to touch the exposed end of the device. Place vial of CINRYZE on a flat surface. Invert diluent vial containing 5 mL Sterile Water for Injection, USP, and insert the clear end into the CINRYZE vial, pushing down until the spike penetrates the rubber stopper and the device snaps into place. The Mix2Vial transfer device must be positioned completely vertical prior to penetrating the stopper closure. The Sterile Water for Injection, USP will automatically flow into the vial of CINRYZE (Figure 3), because the vacuum in the vial will draw in the diluent. If there is no vacuum in the vial, do not use the product. Gently swirl (do not shake) the CINRYZE vial until all powder is dissolved. Be sure that CINRYZE is completely dissolved (Figure 4). Disconnect the Sterile Water for Injection, USP vial by turning it counterclockwise (Figure 5). Do not remove the clear end of the Mix2Vial transfer device from the vial of CINRYZE.One vial of reconstituted CINRYZE contains 5 mL of C1 esterase inhibitor at a concentration of 100 Units/mL. Reconstitute two vials of CINRYZE for one dose. Repeat steps 1 to 9 above using an additional package containing a Mix2Vial transfer device to reconstitute the second of two vials of CINRYZE. Do not reuse the Mix2Vial transfer device. CINRYZE must be administered at room temperature within 3 hours after reconstitution.
2.4 AdministrationTwo vials of reconstituted CINRYZE are combined for a single dose.
Use Aseptic Technique. After reconstitution, the solution should be clear with no evidence of turbidity. Reconstituted solution should be colorless to slightly blue. Do not use if solution is turbid or otherwise discolored. Please refer to the illustrations in steps 7 to 9 included within the Patient Information Leaflet. Utilizing a sterile, disposable 10 mL syringe, draw back the plunger to admit 5 mL air into the syringe. Attach the syringe onto the top of the clear end of the Mix2Vial transfer device by turning it clockwise. Invert the vial and inject air into the solution and then slowly withdraw the reconstituted CINRYZE into the syringe. Detach the syringe from the vial by turning it counterclockwise and releasing it from the clear end of the Mix2Vial transfer device. Using the same syringe, repeat steps 3to 6 with a second vial of CINRYZE to make the complete dose. CINRYZE should be administered promptly after preparation in the syringe and should not be used if particles are observed or if the solution is turbid. Attach a suitable needle or infusion set with winged adapter, and inject intravenously. As a guideline, administer 1,000 Units (reconstituted in 10 mL) of CINRYZE by intravenous injection at a rate of 1 mL per minute over 10 minutes. (see DOSAGE AND ADMINISTRATION [2].) Please refer to the illustration in step 3 of the self administration section within the Patient Information Leaflet. Dispose of all unused solution, the empty vial(s), and the used needles and syringes in an appropriate container for throwing away waste that might hurt others if not handled properly.
Login To Your Free Account