FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Dolorex Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
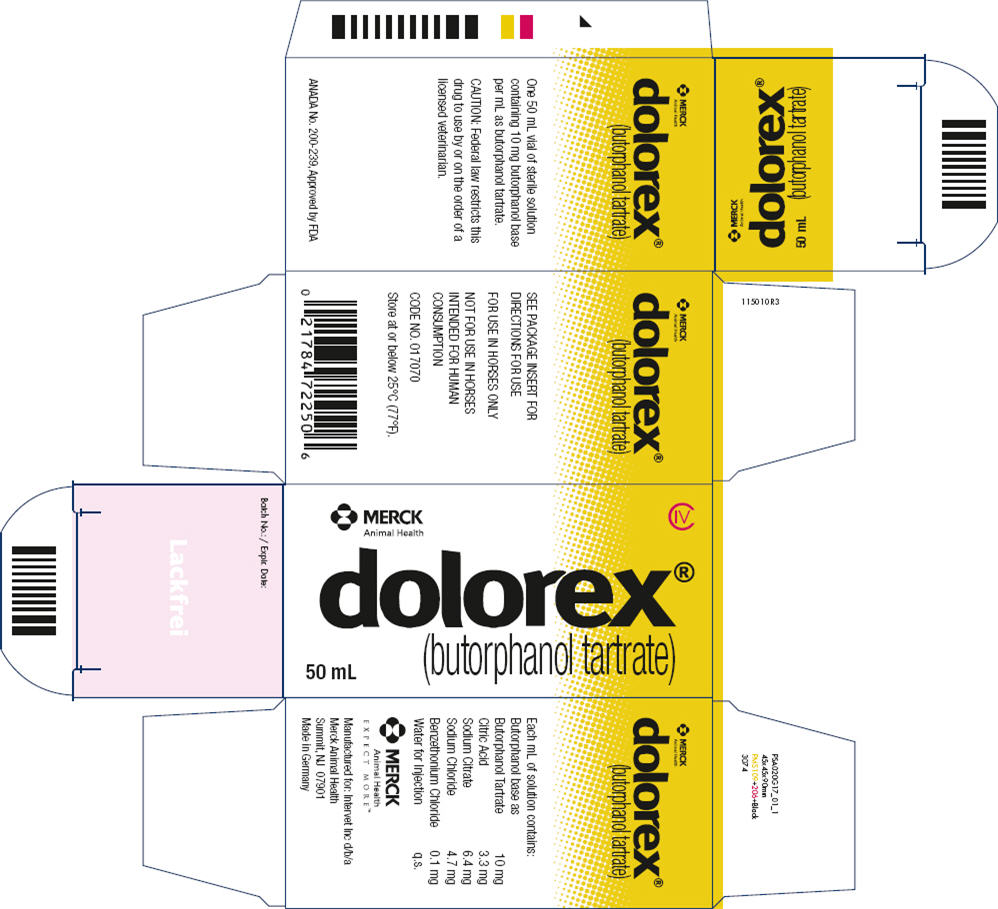
FOR USE IN HORSES ONLY. NOT FOR USE IN HORSES INTENDED FOR HUMAN CONSUMPTION.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
DOLOREX (butorphanol tartrate) is indicated for the relief of pain associated with colic in adult horses and yearlings. Clinical studies in the horse have shown that butorphanol tartrate alleviates abdominal pain associated with torsion, impaction, intussusception, spasmodic and tympanic colic, and postpartum pain.
History
There is currently no drug history available for this drug.
Other Information
DOLOREX (butorphanol tartrate) is a totally synthetic, centrally acting, narcotic agonist-antagonist analgesic with potent antitussive activity. It is a member of the phenanthrene series. The chemical name is Morphinan-3, 14-diol, 17-(cyclobutylmethyl)-, (-)-, (S- (R*, R*))- 2, 3- dihydroxybutanedioate (1:1) (salt). It is a white crystalline, water soluble substance having a molecular weight of 477.55; its molecular formula is C21H29NO2 C4H6O6.

Each mL of DOLOREX contains 10 mg butorphanol base (as butorphanol tartrate, USP), 3.3 mg citric acid, Ph.Eur., 6.4 mg sodium citrate, Ph.Eur., 4.7 mg sodium chloride, Ph.Eur., and 0.1 mg benzethonium chloride, Ph.Eur., q.s. with water for injection, Ph.Eur.
Sources
Dolorex Manufacturers
-
Merck Sharp & Dohme Corp.
![Dolorex (Butorphanol Tartrate) Injection [Merck Sharp & Dohme Corp.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Dolorex | Merck Sharp & Dohme Corp.
![Dolorex (Butorphanol Tartrate) Injection [Merck Sharp & Dohme Corp.] Dolorex (Butorphanol Tartrate) Injection [Merck Sharp & Dohme Corp.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
The recommended dosage in the horse is 0.1 mg butorphanol per kilogram of body weight (0.05 mg/lb) by intravenous injection. This is equivalent to 5 mL DOLOREX for each 1000 lb body weight. The dose may be repeated within 3 to 4 hours but treatment should not exceed 48 hours. Preclinical model studies and clinical field trials in horses demonstrate that the analgesic effects of butorphanol are seen within 15 minutes following injection and persist for about 4 hours.
Login To Your Free Account