FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Enbrel Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Enbrel is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active rheumatoid arthritis (RA). Enbrel can be initiated in combination with methotrexate (MTX) or used alone.
Enbrel is indicated for reducing signs and symptoms of moderately to severely active polyarticular juvenile idiopathic arthritis (JIA) in patients ages 2 and older.
Enbrel is indicated for reducing signs and symptoms, inhibiting the progression of structural damage of active arthritis, and improving physical function in patients with psoriatic arthritis (PsA). Enbrel can be used with or without MTX.
Enbrel is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis (AS).
Enbrel is indicated for the treatment of adult patients (18 years or older) with chronic moderate to severe plaque psoriasis (PsO) who are candidates for systemic therapy or phototherapy.
History
There is currently no drug history available for this drug.
Other Information
Enbrel (etanercept) is a dimeric fusion protein consisting of the extracellular ligand-binding portion of the human 75 kilodalton (p75) tumor necrosis factor receptor (TNFR) linked to the Fc portion of human IgG1. The Fc component of etanercept contains the CH2 domain, the CH3 domain and hinge region, but not the CH1 domain of IgG1. Etanercept is produced by recombinant DNA technology in a Chinese hamster ovary (CHO) mammalian cell expression system. It consists of 934 amino acids and has an apparent molecular weight of approximately 150 kilodaltons.
The solution of Enbrel in the single-use prefilled syringe and the single-use prefilled SureClick autoinjector is clear and colorless, sterile, preservative-free, and is formulated at pH 6.3 ± 0.2.
Enbrel is also supplied in a multiple-use vial as a sterile, white, preservative-free, lyophilized powder. Reconstitution with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (containing 0.9% benzyl alcohol) yields a multiple-use, clear, and colorless solution with a pH of 7.4 ± 0.3.
| Presentation | Active Ingredient Content | Inactive Ingredients Content |
| Enbrel 50 mg prefilled syringe and SureClick autoinjector | 0.98 mL of a 50 mg/mL solution of etanercept | 1% sucrose 100 mM sodium chloride 25 mM L-arginine hydrochloride 25 mM sodium phosphate |
| Enbrel 25 mg prefilled syringe | 0.51 mL of a 50 mg/mL solution of etanercept | 1% sucrose 100 mM sodium chloride 25 mM L-arginine hydrochloride 25 mM sodium phosphate |
| Enbrel 25 mg multiple-use vial | 25 mg etanercept | 40 mg mannitol 10 mg sucrose 1.2 mg tromethamine |
Sources
Enbrel Manufacturers
-
Immunex Corporation
![Enbrel (Etanercept) Solution Enbrel (Etanercept) Kit [Immunex Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Enbrel | Immunex Corporation
![Enbrel (Etanercept) Solution Enbrel (Etanercept) Kit [Immunex Corporation] Enbrel (Etanercept) Solution Enbrel (Etanercept) Kit [Immunex Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Table 1. Dosing and Administration for Adult Patients Patient Population Recommended Dosage Strength and Frequency Adult RA, AS, and PsA Patients 50 mg weekly Adult PsO Patients Starting Dose: 50 mg twice weekly for 3 monthsMaintenance Dose: 50 mg once weekly
See the Enbrel(etanercept) “Instructions for Use” insert for detailed information on injection site selection and dose administration [see Dosage and Administration (2.4) and Patient Counseling Information (17.2)].
2.1 Adult Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis PatientsMTX, glucocorticoids, salicylates, nonsteroidal anti-inflammatory drugs (NSAIDs), or analgesics may be continued during treatment with Enbrel.
Based on a study of 50 mg Enbrel twice weekly in patients with RA that suggested higher incidence of adverse reactions but similar American College of Rheumatology (ACR) response rates, doses higher than 50 mg per week are not recommended.
2.2 Adult Plaque Psoriasis PatientsIn addition to the 50 mg twice weekly recommended starting dose, starting doses of 25 mg or 50 mg per week were shown to be efficacious. The proportion of responders was related to Enbrel dosage [see Clinical Studies (14.5)].
2.3 JIA Patients Table 2. Dosing and Administration for Juvenile Idiopathic Arthritis Pediatric Patients Weight Recommended Dose 63 kg (138 pounds) or more 50 mg weekly Less than 63 kg (138 pounds) 0.8 mg/kg weeklyIn JIA patients, glucocorticoids, NSAIDs, or analgesics may be continued during treatment with Enbrel. Higher doses of Enbrel have not been studied in pediatric patients.
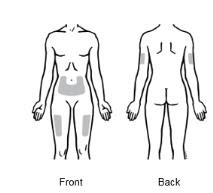
2.4 Preparation of EnbrelEnbrel is intended for use under the guidance and supervision of a physician. Patients may self‑inject when deemed appropriate and if they receive medical follow‑up, as necessary. Patients should not self‑administer until they receive proper training in how to prepare and administer the correct dose. Injections should occur in the thigh, abdomen or outer area of the upper arm.
The Enbrel (etanercept) “Instructions for Use” insert for each presentation contains more detailed instructions on injection site selection and the preparation of Enbrel.
Preparation of Enbrel Using the Single-use Prefilled Syringe
For a more comfortable injection, leave Enbrel at room temperature for about 15 to 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
When using the Enbrel single-use prefilled syringe, check to see if the amount of liquid in the prefilled syringe falls between the two purple fill level indicator lines on the syringe. If the syringe does not have the right amount of liquid, DO NOT USE THAT SYRINGE.
Preparation of Enbrel Using the Single-use Prefilled SureClick Autoinjector
Leave the autoinjector at room temperature for at least 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
Preparation of Enbrel Using the Multiple-use Vial
Enbrel should be reconstituted aseptically with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol), giving a solution of 1.0 mL containing 25 mg of Enbrel.A vial adapter is supplied for use when reconstituting the lyophilized powder. However, the vial adapter should not be used if multiple doses are going to be withdrawn from the vial. If the vial will be used for multiple doses, a 25‑gauge needle should be used for reconstituting and withdrawing Enbrel, and the supplied “Mixing Date:” sticker should be attached to the vial and the date of reconstitution entered. Reconstituted solution must be refrigerated at 36°F to 46°F (2°C to 8°C) and used within 14 days. Discard reconstituted solution after 14 days because product stability and sterility cannot be assured after 14 days. DO NOT store reconstituted Enbrel solution at room temperature.
For a more comfortable injection, leave the Enbrel dose tray at room temperature for about 15 to 30 minutes before injecting.
If using the vial adapter, twist the vial adapter onto the diluent syringe. Then, place the vial adapter over the Enbrel vial and insert the vial adapter into the vial stopper. Push down on the plunger to inject the diluent into the Enbrel vial. If using a 25‑gauge needle to reconstitute and withdraw Enbrel, the diluent should be injected very slowly into the Enbrel vial. It is normal for some foaming to occur. Keeping the diluent syringe in place, gently swirl the contents of the Enbrel vial during dissolution. To avoid excessive foaming, do not shake or vigorously agitate.
Generally, dissolution of Enbrel takes less than 10 minutes. Do not use the solution if discolored or cloudy, or if particulate matter remains.
Withdraw the correct dose of reconstituted solution into the syringe. Some foam or bubbles may remain in the vial. Remove the syringe from the vial adapter or remove the 25‑gauge needle from the syringe. Attach a 27‑gauge needle to inject Enbrel.
The contents of one vial of Enbrel solution should not be mixed with, or transferred into, the contents of another vial of Enbrel. No other medications should be added to solutions containing Enbrel, and do not reconstitute Enbrel with other diluents. Do not filter reconstituted solution during preparation or administration.
2.5 Monitoring to Assess SafetyPrior to initiating Enbrel and periodically during therapy, patients should be evaluated for active tuberculosis and tested for latent infection [see Warnings and Precautions (5.1)].
Login To Your Free Account