FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Esomeprazole Strontium Recall
Get an alert when a recall is issued.
Questions & Answers
Question
Please try and update the date that the medication is to become, or became commercially available at the pharmacy. Such as the newly released generic for Nexium, esomeprazole; it would be nice to know the FDA approval and also commercially available dates. Thanks!
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Healing of Erosive Esophagitis
Esomeprazole strontium is indicated for the short-term treatment (4 to 8 weeks) in the healing and symptomatic resolution of diagnostically confirmed erosive esophagitis. For those patients who have not healed after 4 to 8 weeks of treatment, an additional 4 to 8 week course of esomeprazole strontium may be considered.
Maintenance of Healing of Erosive Esophagitis
Esomeprazole strontium is indicated to maintain symptom resolution and healing of erosive esophagitis. Controlled studies do not extend beyond 6 months.
Symptomatic Gastroesophageal Reflux Disease
Esomeprazole strontium is indicated for short-term treatment (4 to 8 weeks) of heartburn and other symptoms associated with GERD in adults.
Esomeprazole strontium is indicated for the reduction in the occurrence of gastric ulcers associated with continuous NSAID therapy in patients at risk for developing gastric ulcers. Patients are considered to be at risk either due to their age (≥60) and/or documented history of gastric ulcers. Controlled studies do not extend beyond 6 months.
Triple Therapy (esomeprazole strontium plus amoxicillin and clarithromycin): esomeprazole strontium, in combination with amoxicillin and clarithromycin, is indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or history of within the past 5 years) to eradicate H. pylori. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence [see Dosage and Administration (2) and Clinical Studies (14)].
In patients who fail therapy, susceptibility testing should be done. If resistance to clarithromycin is demonstrated or susceptibility testing is not possible, alternative antimicrobial therapy should be instituted [see Clinical Pharmacology (12.4) and the prescribing information for clarithromycin].
Esomeprazole strontium is indicated for the long-term treatment of pathological hypersecretory conditions, including Zollinger-Ellison Syndrome.
History
There is currently no drug history available for this drug.
Other Information
The active ingredient in the proton pump inhibitor esomeprazole strontium delayed-release capsules is bis(5‑ methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole-1-yl) strontium tetrahydrate. Esomeprazole is the S-isomer of omeprazole, which is a mixture of the S- and R- isomers. The molecular formula of esomeprazole strontium is (C17H18N3O3S)2·Sr·4H2O with molecular weight of 848.50. The structural formula is:
Figure 1

The strontium salt is a white or almost white crystalline powder. Each molecule contains 4 moles of water of solvation and is soluble in water.
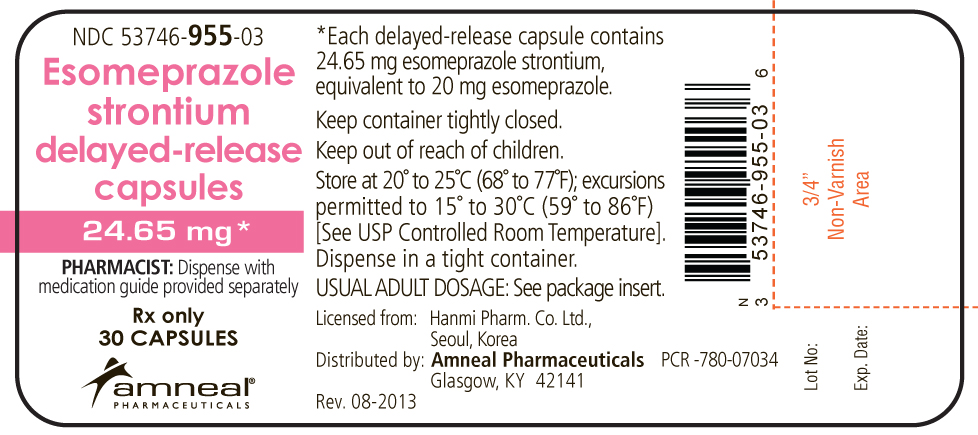
Esomeprazole strontium is supplied in delayed-release capsules. Each delayed-release capsule contains 24.65 mg esomeprazole strontium equivalent to 20 mg esomeprazole or 49.3 mg esomeprazole strontium equivalent to 40 mg esomeprazole, in the form of enteric-coated granules with the following inactive ingredients: calcium carbonate, hypromellose, methacrylic acid copolymer dispersion, mono- and diglycerides, polysorbate 80, sugar spheres, talc, triethyl citrate. The 24.65 mg capsule shells have the following inactive ingredients: gelatin, titanium dioxide, synthetic iron oxide. The 49.3 mg capsule shells have the following inactive ingredients: gelatin, titanium dioxide, FD&C Blue #1, FD&C Red #40, FD&C Yellow #6.
Each 24.65 mg capsule contains 2.6 mg of strontium. Each 49.3 mg capsule contains 5.1 mg of strontium.
Sources
Esomeprazole Strontium Manufacturers
-
Amneal Pharmaceuticals, Llc
![Esomeprazole Strontium Capsule, Delayed Release [Amneal Pharmaceuticals, Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Esomeprazole Strontium | Amneal Pharmaceuticals, Llc
![Esomeprazole Strontium Capsule, Delayed Release [Amneal Pharmaceuticals, Llc] Esomeprazole Strontium Capsule, Delayed Release [Amneal Pharmaceuticals, Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Esomeprazole strontium is supplied as delayed-release capsules for oral administration. The recommended dosages are outlined in Table 1. Esomeprazole strontium should be taken at least one hour before meals.
The duration of proton pump inhibitor administration should be based on available safety and efficacy data specific to the defined indication and dosing frequency, as described in the prescribing information, and individual patient medical needs. Proton pump inhibitor treatment should only be initiated and continued if the benefits outweigh the risks of treatment.
Table 1: Recommended Dosage Schedule of Esomeprazole Strontium Delayed-Release CapsulesIndication
Dose
Frequency
Gastroesophageal Reflux Disease (GERD) in Adults
Healing of Erosive Esophagitis
24.65 mga or 49.3 mgb
Once Daily for 4 to 8 Weeks*
Maintenance of Healing of Erosive Esophagitis
24.65 mga
Once Daily**
Symptomatic Gastroesophageal Reflux Disease
24.65 mga
Once Daily for 4 Weeks***
Risk Reduction of NSAID-Associated Gastric Ulcer in Adults
24.65 mga or 49.3 mgb
Once Daily for up to 6 months**
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence in Adults
Triple Therapy:
esomeprazole strontium
49.3 mgb
Once Daily for 10 Days
Amoxicillin
1000 mg
Twice Daily for 10 Days
Clarithromycin
500 mg
Twice Daily for 10 Days
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome in Adults
49.3 mg†b
‡Twice Daily
*[See Clinical Studies (14.1).] The majority of patients are healed within 4 to 8 weeks. For patients who do not heal after 4 to 8 weeks, an additional 4 to 8 weeks of treatment may be considered.**Controlled studies did not extend beyond six months.
***If symptoms do not resolve completely after 4 weeks, an additional 4 weeks of treatment may be considered. †The dosage of esomeprazole strontium in patients with pathological hypersecretory conditions varies with the individual patient. Dosage regimens should be adjusted to individual patient needs. ‡Doses up to 240 mg daily have been administered [see Drug Interactions (7)]. a 24.65 mg of esomeprazole strontium is equivalent to 20 mg of esomeprazole b 49.3 mg of esomeprazole strontium is equivalent to 40 mg of esomeprazoleRefer to amoxicillin and clarithromycin full prescribing information for Contraindications, Warnings, and dosing in elderly and in renally-impaired patients.
Special Populations
Hepatic Insufficiency
In patients with mild to moderate liver impairment (Child Pugh Classes A and B), no dosage adjustment is necessary. For patients with severe liver impairment (Child Pugh Class C), a dose of 24.65 mg of esomeprazole strontium (equivalent to 20 mg of esomeprazole) should not be exceeded [see Clinical Pharmacology (12.3)].
Administrative Options
Directions for use specific to the route and available methods of administration are presented in Table 2.
Table 2: Administration OptionsAdministration Options
(See text following table for additional instructions.)Dosage Form
Route
Options
Delayed-Release Capsules
Oral
Capsule can be swallowed whole. Do not chew or crush; or
Capsule can be opened and granules mixed with applesauce. Do not chew or crush granules.
Delayed-Release Capsules
Nasogastric Tube
Capsule can be opened and the intact granules emptied into a catheter tipped syringe and delivered through the nasogastric tube.
Esomeprazole strontium delayed-release capsules should be swallowed whole. Do not chew or crush capsule.
Alternatively, for patients who have difficulty swallowing capsules, one tablespoon of applesauce can be added to an empty bowl and the esomeprazole strontium delayed-release capsule can be opened, and the granules inside the capsule carefully emptied onto the applesauce. The granules should be mixed with the applesauce and then swallowed immediately: do not store for future use. The applesauce used should not be hot and should be soft enough to be swallowed without chewing. The granules should not be chewed or crushed. If the granules/applesauce mixture is not used in its entirety, the remaining mixture should be discarded immediately.
For patients who have a nasogastric tube in place, esomeprazole strontium delayed-release capsules can be opened and the intact granules emptied into a 60 mL catheter tipped syringe and mixed with 50 mL of water. It is important to only use a catheter tipped syringe when administering esomeprazole strontium delayed-release capsules through a nasogastric tube. Replace the plunger and shake the syringe vigorously for 15 seconds. Hold the syringe with the tip up and check for granules remaining in the tip. Attach the syringe to a nasogastric tube and deliver the contents of the syringe through the nasogastric tube into the stomach. After administering the granules, the nasogastric tube should be flushed with additional water. Do not administer the granules if they have dissolved or disintegrated.
The mixture must be used immediately after preparation.
-
Amneal Pharmaceuticals Of New York, Llc
![Esomeprazole Strontium Capsule, Delayed Release [Amneal Pharmaceuticals Of New York, Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Esomeprazole Strontium | Amneal Pharmaceuticals Of New York, Llc
![Esomeprazole Strontium Capsule, Delayed Release [Amneal Pharmaceuticals Of New York, Llc] Esomeprazole Strontium Capsule, Delayed Release [Amneal Pharmaceuticals Of New York, Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Esomeprazole strontium is supplied as delayed-release capsules for oral administration. The recommended dosages are outlined in Table 1. Esomeprazole strontium should be taken at least one hour before meals.
The duration of proton pump inhibitor administration should be based on available safety and efficacy data specific to the defined indication and dosing frequency, as described in the prescribing information, and individual patient medical needs. Proton pump inhibitor treatment should only be initiated and continued if the benefits outweigh the risks of treatment.
Table 1: Recommended Dosage Schedule of Esomeprazole Strontium Delayed-Release CapsulesIndication
Dose
Frequency
Gastroesophageal Reflux Disease (GERD) in Adults‡‡
Healing of Erosive Esophagitis
24.65 mga or 49.3 mgb
Once Daily for 4 to 8 Weeks*
Maintenance of Healing of Erosive Esophagitis
24.65 mga
Once Daily**
Symptomatic Gastroesophageal Reflux Disease
24.65 mga
Once Daily for 4 Weeks***
Risk Reduction of NSAID-Associated Gastric Ulcer in Adults
24.65 mga or 49.3 mgb
Once Daily for up to 6 months**
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence in Adults
Triple Therapy:
esomeprazole strontium
49.3 mgb
Once Daily for 10 Days
Amoxicillin
1000 mg
Twice Daily for 10 Days
Clarithromycin
500 mg
Twice Daily for 10 Days
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome in Adults
49.3 mg†b
‡Twice Daily
*[See Clinical Studies (14.1).] The majority of patients are healed within 4 to 8 weeks. For patients who do not heal after 4 to 8 weeks, an additional 4 to 8 weeks of treatment may be considered.**Controlled studies did not extend beyond six months.
***If symptoms do not resolve completely after 4 weeks, an additional 4 weeks of treatment may be considered. †The dosage of esomeprazole strontium in patients with pathological hypersecretory conditions varies with the individual patient. Dosage regimens should be adjusted to individual patient needs. ‡Doses up to 240 mg daily have been administered [see Drug Interactions (7)]. a 24.65 mg of esomeprazole strontium is equivalent to 20 mg of esomeprazole b 49.3 mg of esomeprazole strontium is equivalent to 40 mg of esomeprazole ‡‡Studied for 12 months for Maintenance of Healing of Erosive EsophagitisRefer to amoxicillin and clarithromycin full prescribing information for Contraindications, Warnings, and dosing in elderly and in renally-impaired patients.
Special Populations
Hepatic Insufficiency
In patients with mild to moderate liver impairment (Child Pugh Classes A and B), no dosage adjustment is necessary. For patients with severe liver impairment (Child Pugh Class C), a dose of 24.65 mg of esomeprazole strontium (equivalent to 20 mg of esomeprazole) should not be exceeded [see Clinical Pharmacology (12.3)].
Administrative Options
Directions for use specific to the route and available methods of administration are presented in Table 2.
Table 2: Administration OptionsAdministration Options
(See text following table for additional instructions.)Dosage Form
Route
Options
Delayed-Release Capsules
Oral
Capsule can be swallowed whole. Do not chew or crush; or
Capsule can be opened and granules mixed with applesauce. Do not chew or crush granules.
Delayed-Release Capsules
Nasogastric Tube
Capsule can be opened and the intact granules emptied into a catheter tipped syringe and delivered through the nasogastric tube.
Esomeprazole strontium delayed-release capsules should be swallowed whole. Do not chew or crush capsule.
Alternatively, for patients who have difficulty swallowing capsules, one tablespoon of applesauce can be added to an empty bowl and the esomeprazole strontium delayed-release capsule can be opened, and the granules inside the capsule carefully emptied onto the applesauce. The granules should be mixed with the applesauce and then swallowed immediately: do not store for future use. The applesauce used should not be hot and should be soft enough to be swallowed without chewing. The granules should not be chewed or crushed. If the granules/applesauce mixture is not used in its entirety, the remaining mixture should be discarded immediately.
For patients who have a nasogastric tube in place, esomeprazole strontium delayed-release capsules can be opened and the intact granules emptied into a 60 mL catheter tipped syringe and mixed with 50 mL of water. It is important to only use a catheter tipped syringe when administering esomeprazole strontium delayed-release capsules through a nasogastric tube. Replace the plunger and shake the syringe vigorously for 15 seconds. Hold the syringe with the tip up and check for granules remaining in the tip. Attach the syringe to a nasogastric tube and deliver the contents of the syringe through the nasogastric tube into the stomach. After administering the granules, the nasogastric tube should be flushed with additional water. Do not administer the granules if they have dissolved or disintegrated.
The mixture must be used immediately after preparation.
Login To Your Free Account


![Esomeprazole Strontium Capsule, Delayed Release [Amneal Pharmaceuticals Of New York, Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=61a5a84e-0194-4f11-91f8-83d7ac404932&name=esomeprazole-strontium-delayed-release-capsules-6.jpg)