FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Eucerin Q10 Anti-wrinkle Sensitive Skin Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases
your risk of skin cancer and early skin aging. This product has
been shown only to help prevent sunburn,
not skin cancer or early
skin aging.
For external use only
Do not use on damaged or broken skin.
Stop use and ask a doctor if irritation occurs.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Uses • helps prevent sunburn
History
There is currently no drug history available for this drug.
Other Information
There are no additional details available for this product.
Sources
Eucerin Q10 Anti-wrinkle Sensitive Skin Manufacturers
-
Beiersdorf Inc
![Eucerin Q10 Anti-wrinkle Sensitive Skin (Octinoxate, Octisalate, Oxybenzone) Lotion [Beiersdorf Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Eucerin Q10 Anti-wrinkle Sensitive Skin | Janssen Pharmaceuticals, Inc.
![Eucerin Q10 Anti-wrinkle Sensitive Skin (Octinoxate, Octisalate, Oxybenzone) Lotion [Beiersdorf Inc] Eucerin Q10 Anti-wrinkle Sensitive Skin (Octinoxate, Octisalate, Oxybenzone) Lotion [Beiersdorf Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
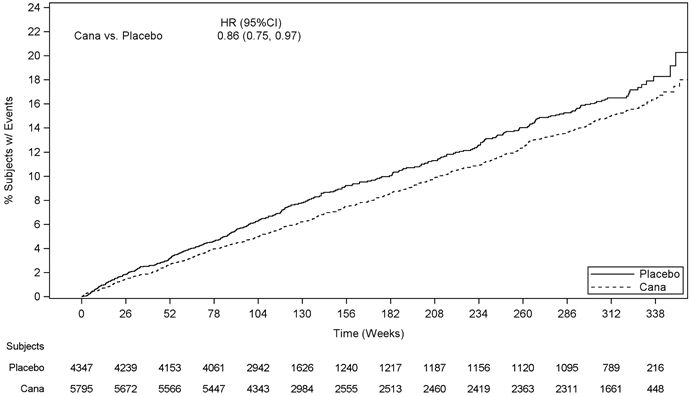
2.1 Recommended Dosage Individualize the starting dose of INVOKAMET (canagliflozin and metformin hydrochloride) based on the patient's current regimen: – In patients on metformin, switch to INVOKAMET containing canagliflozin 50 mg with a similar total daily dose of metformin; – In patients on canagliflozin, switch to INVOKAMET containing metformin 500 mg with a similar total daily dose of canagliflozin; – In patients already treated with canagliflozin and metformin, switch to INVOKAMET containing the same total daily doses of each component. Take INVOKAMET twice daily with meals, with gradual dose escalation to reduce the gastrointestinal side effects due to metformin [see Dosage Forms and Strengths (3)]. In patients with volume depletion not previously treated with canagliflozin, correct this condition before initiating INVOKAMET [see Warnings and Precautions (5.2), Use in Specific Populations (8.5), (8.6), and Patient Counseling Information (17)]. Adjust dosing based on effectiveness and tolerability while not exceeding the maximum recommended daily dose of metformin 2000 mg and canagliflozin 300 mg in patients with an eGFR of 60 mL/min/1.73 m2 or greater [see Dosage and Administration (2.2)]. 2.2 Recommended Dosage for Patients with Renal Impairment Assess renal function before initiating INVOKAMET and periodically thereafter. Do not initiate or continue INVOKAMET in patients with serum creatinine levels greater than or equal to 1.5 mg/dL for males or 1.4 mg/dL for females. In patients who meet these serum creatinine levels, do not initiate or continue INVOKAMET if eGFR is persistently less than 45 mL/min/1.73 m2 [see Contraindications (4) and Warnings and Precautions (5.3)]. No dose adjustment of INVOKAMET is needed in patients with mild renal impairment (eGFR of 60 mL/min/1.73 m2 or greater). Limit the dose of INVOKAMET to canagliflozin 50 mg twice daily in patients with moderate renal impairment with an eGFR of 45 to less than 60 mL/min/1.73 m2. 2.3 Concomitant Use with UDP-Glucuronosyl Transferase (UGT) Enzyme InducersIf an inducer of UGTs (e.g., rifampin, phenytoin, phenobarbital, ritonavir) is co-administered with INVOKAMET, consider increasing the dose to canagliflozin 150 mg twice daily in patients currently tolerating 50 mg twice daily who have an eGFR of 60 mL/min/1.73 m2 or greater and require additional glycemic control [see Drug Interactions (7.2)].
Consider another antihyperglycemic agent in patients with an eGFR of 45 to less than 60 mL/min/1.73 m2 receiving concurrent therapy with a UGT inducer.
Login To Your Free Account