FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Glytone Suncare Block Sunscreen Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Warnings
Do not use on damaged or broken skin.When using this product keep out of eyes. Rinse with water to remove
Stop use and ask a doctor if rash occurs
If pregnant or breast feeding, ask a health professional before use
For external use only
Keep away from children
Do not swallow. If swallowed, get medical help or contact Poison Control right away.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Uses Helps prevent sunburn
History
There is currently no drug history available for this drug.
Other Information
There are no additional details available for this product.
Sources
Glytone Suncare Block Sunscreen Manufacturers
-
Genesis Pharmaceutical
![Glytone Suncare Block Sunscreen (Octinoxate, Octisalate, Xinc Oxide) Lotion [Genesis Pharmaceutical]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Glytone Suncare Block Sunscreen | Merck Sharp & Dohme Corp.
![Glytone Suncare Block Sunscreen (Octinoxate, Octisalate, Xinc Oxide) Lotion [Genesis Pharmaceutical] Glytone Suncare Block Sunscreen (Octinoxate, Octisalate, Xinc Oxide) Lotion [Genesis Pharmaceutical]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
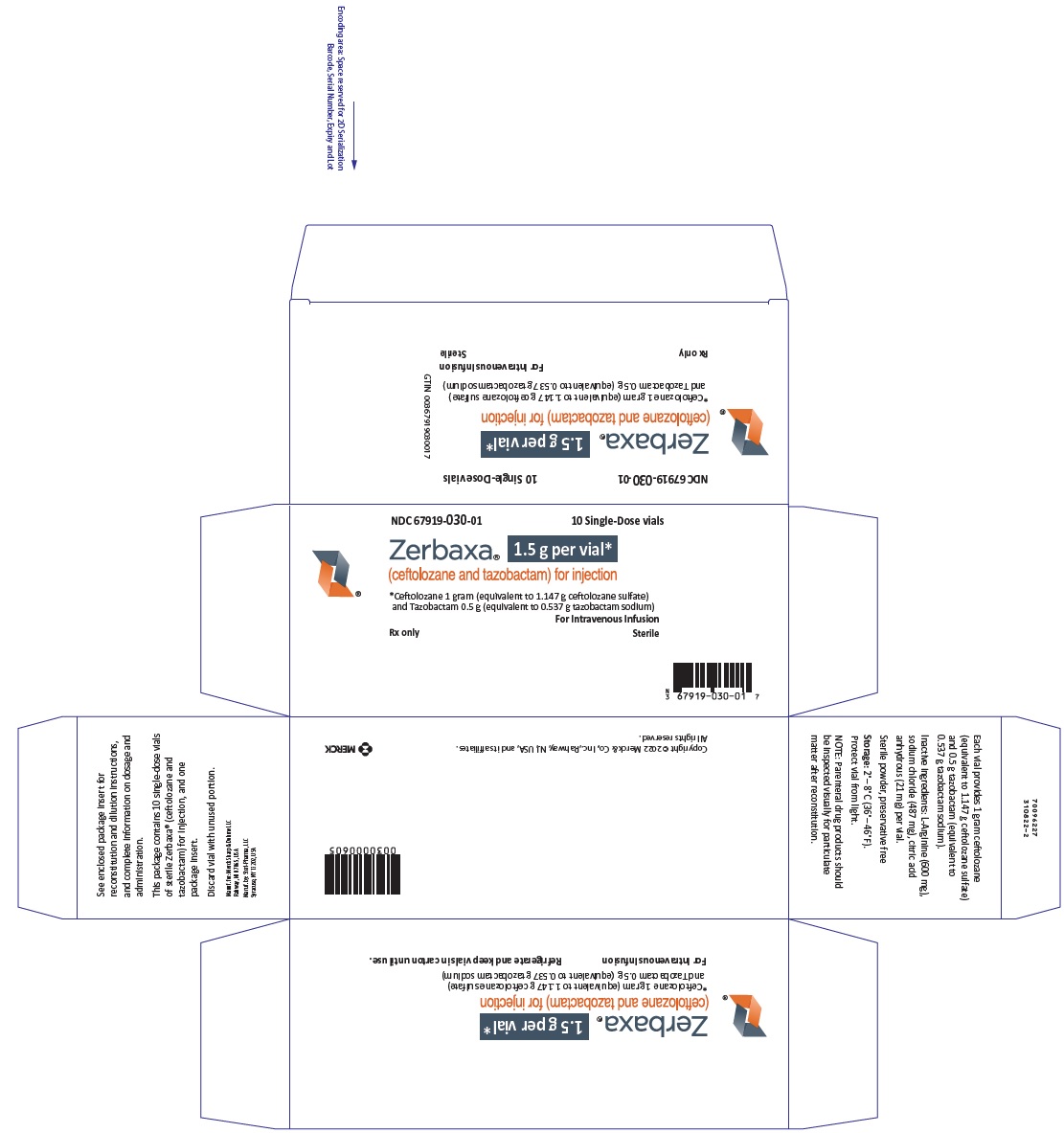
2.1 Recommended DosageThe recommended dosage regimen is ZERBAXA 1.5 gram (g) (ceftolozane 1 g and tazobactam 0.5 g) for injection administered every 8 hours by intravenous infusion over 1 hour in patients 18 years or older and with normal renal function or mild renal impairment. The duration of therapy should be guided by the severity and site of infection and the patient's clinical and bacteriological progress (Table 1).
Table 1: Dosage of ZERBAXA 1.5 g (ceftolozane 1 g and tazobactam 0.5 g) by Infection in Patients with Creatinine Clearance (CrCl) Greater than 50 mL/min Infection Dose Frequency Infusion Time (hours) Duration of Treatment * Used in conjunction with metronidazole 500 mg intravenously every 8 hours Complicated Intra-abdominal Infections* 1.5 g Every 8 Hours 1 4-14 days Complicated Urinary Tract Infections, including Pyelonephritis 1.5 g Every 8 Hours 1 7 days 2.2 Patients with Renal ImpairmentDose adjustment is required for patients whose creatinine clearance is 50 mL/min or less. Renal dose adjustments are listed in Table 2. For patients with changing renal function, monitor CrCl at least daily and adjust the dosage of ZERBAXA accordingly [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Table 2: Dosage of ZERBAXA in Patients with Renal Impairment Estimated CrCl (mL/min)* Recommended Dosage Regimen for ZERBAXA 1.5 g (ceftolozane 1 g and tazobactam 0.5 g)† * CrCl estimated using Cockcroft-Gault formula † All doses of ZERBAXA are administered over 1 hour. 30 to 50 750 mg (500 mg and 250 mg) intravenously every 8 hours 15 to 29 375 mg (250 mg and 125 mg) intravenously every 8 hours End-stage renal disease (ESRD) on hemodialysis (HD) A single loading dose of 750 mg (500 mg and 250 mg) followed by a 150 mg (100 mg and 50 mg) maintenance dose administered every 8 hours for the remainder of the treatment period (on hemodialysis days, administer the dose at the earliest possible time following completion of dialysis) 2.3 Preparation of SolutionsZERBAXA does not contain a bacteriostatic preservative. Aseptic technique must be followed in preparing the infusion solution.
Preparation of doses:
Constitute the vial with 10 mL of sterile water for injection or 0.9% Sodium Chloride for Injection, USP and gently shake to dissolve. The final volume is approximately 11.4 mL. Caution: The constituted solution is not for direct injection.
To prepare the required dose, withdraw the appropriate volume determined from Table 3 from the reconstituted vial. Add the withdrawn volume to an infusion bag containing 100 mL of 0.9% Sodium Chloride for Injection, USP or 5% Dextrose Injection, USP.
Table 3: Preparation of Doses ZERBAXA (ceftolozane and tazobactam) Dose Volume to Withdraw from Reconstituted Vial 1.5 g (1 g and 0.5 g) 11.4 mL (entire contents) 750 mg (500 mg and 250 mg) 5.7 mL 375 mg (250 mg and 125 mg) 2.9 mL 150 mg (100 mg and 50 mg) 1.2 mLInspect drug products visually for particulate matter and discoloration prior to use. ZERBAXA infusions range from clear, colorless solutions to solutions that are clear and slightly yellow. Variations in color within this range do not affect the potency of the product.
2.4 CompatibilityCompatibility of ZERBAXA with other drugs has not been established. ZERBAXA should not be mixed with other drugs or physically added to solutions containing other drugs.
2.5 Storage of Constituted SolutionsUpon constitution with sterile water for injection or 0.9% sodium chloride injection, reconstituted ZERBAXA solution may be held for 1 hour prior to transfer and dilution in a suitable infusion bag.
Following dilution of the solution with 0.9% sodium chloride or 5% dextrose, ZERBAXA is stable for 24 hours when stored at room temperature or 7 days when stored under refrigeration at 2 to 8°C (36 to 46°F).
Constituted ZERBAXA solution or diluted ZERBAXA infusion should not be frozen.
Login To Your Free Account