FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Haldol Decanoate Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. HALDOL Decanoate is not approved for the treatment of patients with dementia-related psychosis (see BOXED WARNING).
Cases of sudden death, QT-prolongation, and Torsades de Pointes have been reported in patients receiving haloperidol. Higher than recommended doses of any formulation and intravenous administration of haloperidol appear to be associated with a higher risk of QT-prolongation and Torsades de Pointes. Although cases have been reported even in the absence of predisposing factors, particular caution is advised in treating patients with other QT-prolonging conditions (including electrolyte imbalance [particularly hypokalemia and hypomagnesemia], drugs known to prolong QT, underlying cardiac abnormalities, hypothyroidism, and familial long QT-syndrome). HALDOL DECANOATE MUST NOT BE ADMINISTERED INTRAVENOUSLY.
A syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing tardive dyskinesia and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, antipsychotic drugs should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that 1) is known to respond to antipsychotic drugs, and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on antipsychotics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome. (For further information about the description of tardive dyskinesia and its clinical detection, please refer to ADVERSE REACTIONS.)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status (including catatonic signs) and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis) and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Hyperpyrexia and heat stroke, not associated with the above symptom complex, have also been reported with HALDOL.
An encephalopathic syndrome (characterized by weakness, lethargy, fever, tremulousness and confusion, extrapyramidal symptoms, leukocytosis, elevated serum enzymes, BUN, and fasting blood sugar) followed by irreversible brain damage has occurred in a few patients treated with lithium plus HALDOL. A causal relationship between these events and the concomitant administration of lithium and HALDOL has not been established; however, patients receiving such combined therapy should be monitored closely for early evidence of neurological toxicity and treatment discontinued promptly if such signs appear.
A number of cases of bronchopneumonia, some fatal, have followed the use of antipsychotic drugs, including HALDOL (haloperidol). It has been postulated that lethargy and decreased sensation of thirst due to central inhibition may lead to dehydration, hemoconcentration and reduced pulmonary ventilation. Therefore, if the above signs and symptoms appear, especially in the elderly, the physician should institute remedial therapy promptly.
Although not reported with HALDOL, decreased serum cholesterol and/or cutaneous and ocular changes have been reported in patients receiving chemically-related drugs.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
HALDOL Decanoate 50 and HALDOL Decanoate 100 are indicated for the treatment of schizophrenic patients who require prolonged parenteral antipsychotic therapy.
History
There is currently no drug history available for this drug.
Other Information
Haloperidol decanoate is the decanoate ester of the butyrophenone, HALDOL (haloperidol). It has a markedly extended duration of effect. It is available in sesame oil in sterile form for intramuscular (IM) injection. The structural formula of haloperidol decanoate, 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]-4 piperidinyl decanoate, is:

Haloperidol decanoate is almost insoluble in water (0.01 mg/mL), but is soluble in most organic solvents.
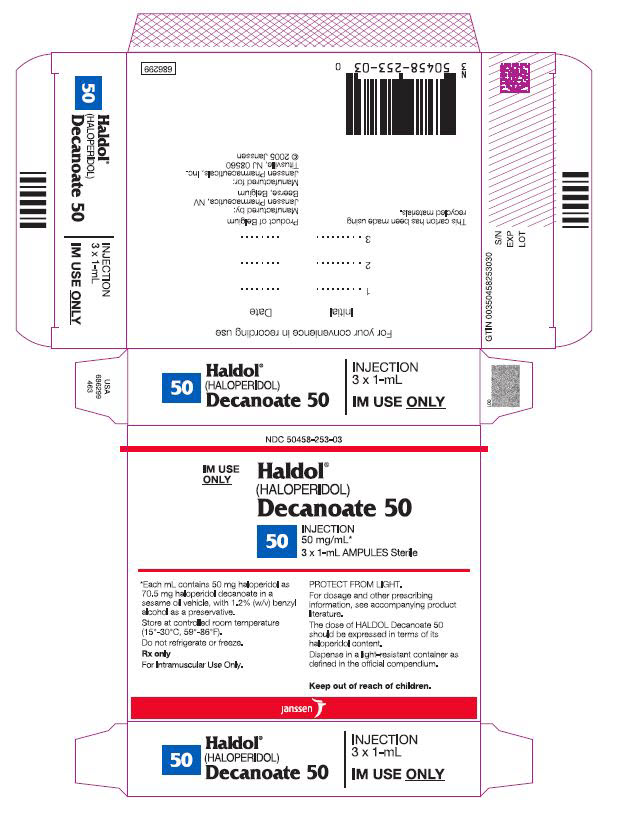
Each mL of HALDOL Decanoate 50 for IM injection contains 50 mg haloperidol (present as haloperidol decanoate 70.52 mg) in a sesame oil vehicle, with 1.2% (w/v) benzyl alcohol as a preservative.
Each mL of HALDOL Decanoate 100 for IM injection contains 100 mg haloperidol (present as haloperidol decanoate 141.04 mg) in a sesame oil vehicle, with 1.2% (w/v) benzyl alcohol as a preservative.
Sources
Haldol Decanoate Manufacturers
-
Janssen Pharmaceuticals, Inc.
![Haldol Decanoate (Haloperidol Decanoate) Injection [Janssen Pharmaceuticals, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Haldol Decanoate | Janssen Pharmaceuticals, Inc.
![Haldol Decanoate (Haloperidol Decanoate) Injection [Janssen Pharmaceuticals, Inc.] Haldol Decanoate (Haloperidol Decanoate) Injection [Janssen Pharmaceuticals, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
HALDOL Decanoate 50 and HALDOL Decanoate 100 should be administered by deep intramuscular injection. A 21 gauge needle is recommended. The maximum volume per injection site should not exceed 3 mL. DO NOT ADMINISTER INTRAVENOUSLY.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HALDOL Decanoate 50 and HALDOL Decanoate 100 are intended for use in schizophrenic patients who require prolonged parenteral antipsychotic therapy. These patients should be previously stabilized on antipsychotic medication before considering a conversion to haloperidol decanoate. Furthermore, it is recommended that patients being considered for haloperidol decanoate therapy have been treated with, and tolerate well, short-acting HALDOL (haloperidol) in order to reduce the possibility of an unexpected adverse sensitivity to haloperidol. Close clinical supervision is required during the initial period of dose adjustment in order to minimize the risk of overdosage or reappearance of psychotic symptoms before the next injection. During dose adjustment or episodes of exacerbation of symptoms of schizophrenia, haloperidol decanoate therapy can be supplemented with short-acting forms of haloperidol.
The dose of HALDOL Decanoate 50 or HALDOL Decanoate 100 should be expressed in terms of its haloperidol content. The starting dose of haloperidol decanoate should be based on the patient's age, clinical history, physical condition, and response to previous antipsychotic therapy. The preferred approach to determining the minimum effective dose is to begin with lower initial doses and to adjust the dose upward as needed. For patients previously maintained on low doses of antipsychotics (e.g. up to the equivalent of 10 mg/day oral haloperidol), it is recommended that the initial dose of haloperidol decanoate be 10–15 times the previous daily dose in oral haloperidol equivalents; limited clinical experience suggests that lower initial doses may be adequate.
Initial TherapyConversion from oral haloperidol to haloperidol decanoate can be achieved by using an initial dose of haloperidol decanoate that is 10 to 20 times the previous daily dose in oral haloperidol equivalents.
In patients who are elderly, debilitated, or stable on low doses of oral haloperidol (e.g. up to the equivalent of 10 mg/day oral haloperidol), a range of 10 to 15 times the previous daily dose in oral haloperidol equivalents is appropriate for initial conversion.
In patients previously maintained on higher doses of antipsychotics for whom a low dose approach risks recurrence of psychiatric decompensation and in patients whose long-term use of haloperidol has resulted in a tolerance to the drug, 20 times the previous daily dose in oral haloperidol equivalents should be considered for initial conversion, with downward titration on succeeding injections.
The initial dose of haloperidol decanoate should not exceed 100 mg regardless of previous antipsychotic dose requirements. If, therefore, conversion requires more than 100 mg of haloperidol decanoate as an initial dose, that dose should be administered in two injections, i.e. a maximum of 100 mg initially followed by the balance in 3 to 7 days.
Maintenance TherapyThe maintenance dosage of haloperidol decanoate must be individualized with titration upward or downward based on therapeutic response. The usual maintenance range is 10 to 15 times the previous daily dose in oral haloperidol equivalents dependent on the clinical response of the patient.
HALDOL DECANOATE DOSING RECOMMENDATIONS Patients Monthly
1st Month Maintenance Stabilized on low daily oral doses
(up to 10 mg/day)
Elderly or Debilitated 10–15 × Daily Oral Dose 10–15 × Previous Daily Oral Dose High dose
Risk of relapse 20 × Daily Oral Dose 10–15 × Previous Daily Oral Dose Tolerant to oral haloperidolClose clinical supervision is required during initiation and stabilization of haloperidol decanoate therapy. Haloperidol decanoate is usually administered monthly or every 4 weeks. However, variation in patient response may dictate a need for adjustment of the dosing interval as well as the dose (See CLINICAL PHARMACOLOGY).
Clinical experience with haloperidol decanoate at doses greater than 450 mg per month has been limited.
Instructions for Opening Ampule
1. Medication often rests in the top part of the ampule. Before breaking the ampule, lightly tap the top of the ampule with your finger until all fluid moves to the bottom portion of the ampule. The ampule has a colored ring(s) and colored point which aids in the placement of fingers while breaking the ampule. Step 1
2. Hold the ampule between thumb and index finger with the colored point facing you. Step 2
3. Position the index finger of the other hand to support the neck of the ampule. Position the thumb so that it covers the colored point and is parallel to the colored ring(s). Step 3
4. Keeping the thumb on the colored point and with the index fingers close together, apply firm pressure on the colored point in the direction of the arrow to snap the ampule open. Step 4
Login To Your Free Account