FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Lucentis Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
LUCENTIS is indicated for the treatment of patients with:
History
There is currently no drug history available for this drug.
Other Information
LUCENTIS® (ranibizumab injection) is a recombinant humanized IgG1 kappa isotype monoclonal antibody fragment designed for intraocular use. Ranibizumab binds to and inhibits the biologic activity of human vascular endothelial growth factor A (VEGF-A). Ranibizumab, which lacks an Fc region, has a molecular weight of approximately 48 kilodaltons and is produced by an E. coli expression system in a nutrient medium containing the antibiotic tetracycline. Tetracycline is not detectable in the final product.
LUCENTIS is a sterile, colorless to pale yellow solution in a single-use glass vial. LUCENTIS is supplied as a preservative-free, sterile solution in a single-use glass vial designed to deliver 0.05 mL of 10 mg/mL LUCENTIS (0.5 mg dose vial) or 6 mg/mL LUCENTIS (0.3 mg dose vial) aqueous solution with 10 mM histidine HCl, 10% α,α-trehalose dihydrate, 0.01% polysorbate 20, pH 5.5.
Sources
Lucentis Manufacturers
-
Genentech, Inc.
![Lucentis (Ranibizumab) Injection, Solution [Genentech, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Lucentis | Genentech, Inc.
![Lucentis (Ranibizumab) Injection, Solution [Genentech, Inc.] Lucentis (Ranibizumab) Injection, Solution [Genentech, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 General Dosing InformationFOR OPHTHALMIC INTRAVITREAL INJECTION ONLY.
2.2 Neovascular (Wet) Age-Related Macular Degeneration (AMD)LUCENTIS 0.5 mg (0.05 mL of 10 mg/mL LUCENTIS solution) is recommended to be administered by intravitreal injection once a month (approximately 28 days).
Although not as effective, patients may be treated with 3 monthly doses followed by less frequent dosing with regular assessment. In the nine months after 3 initial monthly doses, less frequent dosing with 4-5 doses on average is expected to maintain visual acuity while monthly dosing may be expected to result in an additional average 1-2 letter gain. Patients should be assessed regularly [see Clinical Studies (14.1)].
Although not as effective, patients may also be treated with one dose every 3 months after 4 monthly doses. Compared with continued monthly dosing, dosing every 3 months over the next 9 months will lead to an approximate 5-letter (1-line) loss of visual acuity benefit, on average. Patients should be assessed regularly [see Clinical Studies (14.1)].
2.3 Macular Edema Following Retinal Vein Occlusion (RVO)LUCENTIS 0.5 mg (0.05 mL of 10 mg/mL LUCENTIS solution) is recommended to be administered by intravitreal injection once a month (approximately 28 days).
In Studies RVO-1 and RVO-2, patients received monthly injections of LUCENTIS for 6 months. In spite of being guided by optical coherence tomography and visual acuity re-treatment criteria, patients who were then not treated at Month 6 experienced on average, a loss of visual acuity at Month 7, whereas patients who were treated at Month 6 did not. Patients should be treated monthly [see Clinical Studies (14.2)].
2.4 Diabetic Macular Edema (DME)LUCENTIS 0.3 mg (0.05 mL of 6 mg/mL LUCENTIS solution) is recommended to be administered by intravitreal injection once a month (approximately 28 days).
2.5 Diabetic Retinopathy in patients with Diabetic Macular EdemaLUCENTIS 0.3 mg (0.05 mL of 6 mg/mL LUCENTIS solution) is recommended to be administered by intravitreal injection once a month (approximately 28 days).
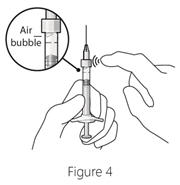
2.6 Preparation for AdministrationUsing aseptic technique, all of the LUCENTIS vial contents are withdrawn through a 5-micron, 19-gauge filter needle attached to a 1-cc tuberculin syringe. The filter needle should be discarded after withdrawal of the vial contents and should not be used for intravitreal injection. The filter needle should be replaced with a sterile 30-gauge × 1/2-inch needle for the intravitreal injection. The contents should be expelled until the plunger tip is aligned with the line that marks 0.05 mL on the syringe.
2.7 AdministrationThe intravitreal injection procedure should be carried out under controlled aseptic conditions, which include the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection.
Prior to and 30 minutes following the intravitreal injection, patients should be monitored for elevation in intraocular pressure using tonometry. Monitoring may also consist of a check for perfusion of the optic nerve head immediately after the injection [see Warnings and Precautions (5.2)]. Patients should also be monitored for and instructed to report any symptoms suggestive of endophthalmitis without delay following the injection [see Warnings and Precautions (5.1)].
Each vial should only be used for the treatment of a single eye. If the contralateral eye requires treatment, a new vial should be used and the sterile field, syringe, gloves, drapes, eyelid speculum, filter, and injection needles should be changed before LUCENTIS is administered to the other eye.
No special dosage modification is required for any of the populations that have been studied (e.g., gender, elderly).
Login To Your Free Account