Minoxidil Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
1. Salt and Water Retention:
Congestive Heart Failure - concomitant use of an adequate diuretic is required - Minoxidil tablets must usually be administered concomitantly with a diuretic adequate to prevent fluid retention and possible congestive heart failure; a high ceiling (loop) diuretic is almost always srequired. Body weight should be monitored closely. If minoxidil is used without a diuretic, retention of several hundred milli-equivalents of salt and corresponding volumes of water can occur within a few days, leading to increased plasma and interstitial fluid volume and local or generalized edema. Diuretic treatment alone, or in combination with restricted salt intake, will usually minimize fluid retention, although reversible edema did develop in approximately 10% of nondialysis patients so treated. Ascites has also been reported. Diuretic effectiveness was limited mostly by disease-related impaired renal function. The condition of patients with pre-existing congestive heart failure occasionally deteriorated in association with fluid retention although because of the fall in blood pressure (reduction of afterload), more than twice as many improved than worsened. Rarely refractory fluid retention may require discontinuation of minoxidil. Provided that the patient is under close medical supervision, it may be possible to resolve refractory salt retention by discontinuing minoxidil for 1 to 2 days and then resuming treatment in conjunction with vigorous diuretic therapy.
2. Concomitant Treatment to Prevent Tachycardia is Usually Required - Minoxidil increases the heart rate. Angina may worsen or appear for the first time during minoxidil treatment, probably because of the increased oxygen demands associated with increased heart rate and cardiac output. The increase in rate and the occurrence of angina generally can be prevented by the concomitant administration of a beta-adrenergic blocking drug or other sympathetic nervous system suppressant. The ability of beta-adrenergic blocking agents to minimize papillary muscle lesions in animals is further reason to utilize such an agent concomitantly. Round-the-clock effectiveness of the sympathetic suppressant should be ensured.
3. Pericarditis, Pericardial Effusion and Tamponade - There have been reports of pericarditis occurring in association with the use of minoxidil. The relationship of this association to renal status is uncertain. Pericardial effusion, occasionally with tamponade, has been observed in about 3% of treated patients not on dialysis, especially those with inadequate or compromised renal function. Although in many cases, the pericardial effusion was associated with a connective tissue disease, the uremic syndrome, congestive heart failure, or marked fluid retention, there have been instances in which these potential causes of effusion were not present. Patients should be observed closely for any suggestion of a pericardial disorder, and echocardiographic studies should be carried out if suspicion arises. More vigorous diuretic therapy, dialysis, pericardiocentesis, or surgery may be required. If the effusion persists, withdrawal of minoxidil should be considered in light of other means of controlling the hypertension and the patient’s clinical status.
4. Interaction with Guanethidine:
Although minoxidil does not itself cause orthostatic hypotension, its administration to patients already receiving guanethidine can result in profound orthostatic effects. If at all possible, guanethidine should be discontinued well before minoxidil is begun. Where this is not possible, minoxidil therapy should be started in the hospital and the patient should remain institutionalized until severe orthostatic effects are no longer present or the patient has learned to avoid activities that provoke them.
5. Hazard of Rapid Control of Blood Pressure:
In patients with very severe blood pressure elevation, too rapid control of blood pressure, especially with intravenous agents, can precipitate syncope, cerebrovascular accidents, myocardial infarction and ischemia of special sense organs with resulting decrease or loss of vision or hearing. Patients with compromised circulation or cryoglobulinemia may also suffer ischemic episodes of the affected organs. Although such events have not been unequivocally associated with minoxidil use, total experience is limited at present.
Any patient with malignant hypertension should have initial treatment with minoxidil carried out in a hospital setting, both to assure that blood pressure is falling and to assure that it is not falling more rapidly than intended.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Because of the potential for serious adverse effects, minoxidil tablets are indicated only in the treatment of hypertension that is symptomatic or associated with target organ damage and is not manageable with maximum therapeutic doses of diuretic plus two other antihypertensive drugs. At the present time use in milder degrees of hypertension is not recommended because the benefit-risk relationship in such patients has not been defined.
Minoxidil reduced supine diastolic blood pressure by 20 mmHg or to 90 mmHg or less in approximately 75% of patients, most of whom had hypertension that could not be controlled by other drugs.
History
There is currently no drug history available for this drug.
Other Information
Minoxidil tablets contain minoxidil, an antihypertensive peripheral vasodilator. Minoxidil occurs as a white to off-white, odorless, crystalline solid that is soluble in water to the extent of approximately 2 mg/mL, is readily soluble in propylene glycol or ethanol, and is almost insoluble in acetone, chloroform or ethyl acetate. The chemical name for minoxidil is 2,4-pyrimidinediamine, 6-(1-piperidinyl)-, 3-oxide.
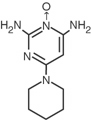
C9H15N50 MW 209.25
Minoxidil tablets for oral administration contain either 2.5 mg or 10 mg of minoxidil. Inactive ingredients include colloidal silicon dioxide, corn starch, lactose anhydrous, magnesium stearate and microcrystalline cellulose.
Sources




![Minoxidil Tablet [Mutual Pharmaceutical Company, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0354a7f5-5917-44dc-9963-411104008cb5&name=minoxidil-16.jpg)
![Minoxidil Tablet [Ncs Healthcare Of Ky, Inc Dba Vangard Labs]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d7f4088e-e99a-4e3e-aea4-2c7834abe7b5&name=minoxidil-10.jpg)
![Minoxidil Tablet [State Of Florida Doh Central Pharmacy]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=abeeb857-8303-487a-83fc-de7396c6ea8b&name=Minoxidil10mg(MutualPharma).jpg)
![Minoxidil Tablet [Watson Laboratories, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=ab30461c-f8c8-409d-9e24-d58ed8a34873&name=minoxidil-tablets-12.jpg)
![Minoxidil Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0e830772-b1d7-4608-972e-b98cdf06327e&name=MM12.jpg)
![Minoxidil Tablet Minoxidil Tablet [Physicians Total Care, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=56274d6f-8439-4ab7-9bb8-a3fc10a26463&name=3465.jpg)
![Minoxidil Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=da4c68cf-e25f-45a7-a749-0b987ec2ba96&name=MM12.jpg)
![Minoxidil Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=7dee5448-515c-4152-8c6b-7571f6068bd2&name=MM16.jpg)
![Minoxidil Tablet [Par Pharmaceutical Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=8f3800f0-b6da-4dfe-8c32-39bb5eb0262a&name=9e2fa5e7-1087-46ea-bf5f-4fd5963b4bb4-12.jpg)
![Minoxidil Tablet [A-s Medication Solutions Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0697e266-f8ab-4ccf-9d84-cc230e7a913a&name=1983-0.jpg)
![Minoxidil Tablet [American Health Packaging]](https://www.recallguide.org/wp-content/themes/bootstrap/assets/img/drug-image-placeholder.jpg)
![Minoxidil (Minoxidil) Tablet [Avkare, Inc.]](http://recallguide.cwdevelopsp.com/wp-content/themes/recallguide/assets/img/drug-image-placeholder.jpg)
![Minoxidil Tablet [Cardinal Health]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=96592d7b-6201-4add-b99d-b42a13f8bd95&name=image-01.jpg)