Neomycin Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
NOT FOR INJECTION INTO THE EYE. Neomycin and Polymyxin B Sulfates and Hydrocortisone Ophthalmic Suspension should never be directly introduced into the anterior chamber of the eye.
Prolonged use of corticosteroids may result in ocular hypertension and/or glaucoma, with damage to the optic nerve, defects in visual acuity and fields of vision, and posterior subcapsular cataract formation.
Prolonged use may suppress the host response and thus increase the hazard of secondary ocular infections. In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical corticosteroids. In acute purulent conditions of the eye, corticosteroids may mask infection or enhance existing infection.
If these products are used for 10 days or longer, intraocular pressure should be routinely monitored even though it may be difficult in uncooperative patients. Corticosteroids should be used with caution in the presence of glaucoma.
The use of corticosteroids after cataract surgery may delay healing and increase the incidence of filtering blebs.
Use of ocular corticosteroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of corticosteroid medication in the treatment of herpes simplex requires great caution.
Topical antibiotics, particularly, neomycin sulfate, may cause cutaneous sensitization. A precise incidence of hypersensitivity reactions (primarily skin rash) due to topical antibiotics is not known. The manifestations of sensitization to topical antibiotics are usually itching, reddening, and edema of the conjunctiva and eyelid. A sensitization reaction may manifest simply as a failure to heal. During long-term use of topical antibiotic products, periodic examination for such signs is advisable, and the patient should be told to discontinue the product if they are observed. Symptoms usually subside quickly on withdrawing the medication. Application of products containing these ingredients should be avoided for the patient thereafter (see PRECAUTIONS: General).
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Neomycin and Polymyxin B Sulfates and Hydrocortisone Ophthalmic Suspension is indicated for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where bacterial infection or a risk of bacterial ocular infection exists.
Ocular corticosteroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe where the inherent risk of corticosteroid use in certain infective conjunctivitides is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation, or thermal burns, or penetration of foreign bodies.
The use of a combination drug with an anti-infective component is indicated where the risk of infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye (See CLINICAL PHARMACOLOGY: Microbiology).
The particular anti-infective drugs in this product are active against the following common bacterial eye pathogens: Staphylococcus aureus, Escherichia coli, Haemophilus influenzae, Klebsiella/Enterobacter species, Neisseria species, and Pseudomonas aeruginosa.
The product does not provide adequate coverage against Serratia marcescens and streptococci, including Streptococcus pneumoniae.
History
There is currently no drug history available for this drug.
Other Information
Neomycin and Polymyxin B Sulfates and Hydrocortisone Ophthalmic Suspension is a sterile antimicrobial and anti-inflammatory suspension for ophthalmic use.
Each mL contains: Actives: neomycin sulfate (equivalent to 3.5 mg neomycin base), polymyxin B sulfate equivalent to 10,000 polymyxin B units, and hydrocortisone 10 mg (1%). Preservative: thimerosal 0.001%. Inactives: cetyl alcohol, glyceryl monostearate, mineral oil, polyoxyl 40 stearate, propylene glycol, sulfuric acid (to adjust pH) and water for injection.
Neomycin sulfate is the sulfate salt of neomycin B and C, which are produced by the growth of Streptomyces fradiae Waksman (Fam. Streptomycetacae). It has a potency equivalent to not less than 600 μg of neomycin standard per mg, calculated on an anhydrous basis. Its structural formula are:

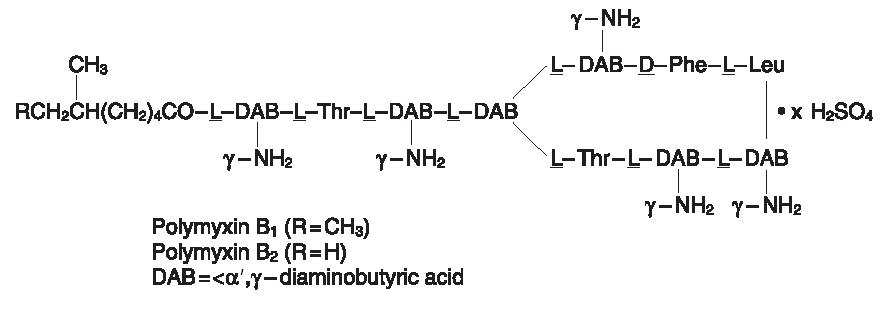
Polymyxin B sulfate is the sulfate salt of Polymyxin, B1 and B2, which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per mg calculated on an anhydrous basis. Its structural formula are:
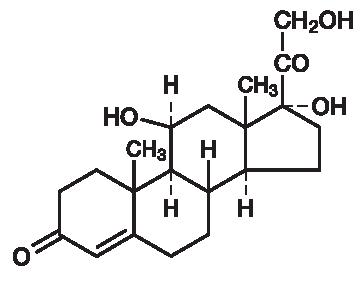
Hydrocortisone, 11β, 17, 21-trihydroxypregn-4-ene-3, 20 dione, is an anti-inflammatory hormone. Its structural formula is:
Sources






![Neomycin Liquid [Sparhawk Laboratories, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=b46a696a-c49e-406a-90fa-8e06eff9d231&name=NeomycinLiquid-12.jpg)
![Neomycin Liquid [Agri Laboratories, Ltd.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=96875301-0446-493b-8274-d46c57e09a70&name=AL-NeomycinOral-12.jpg)
![Neomycin Liquid [Durvet, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=91040196-6998-4e3e-9718-878d620b541f&name=DV-NeomycinOral-12.jpg)