FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Neulasta Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Neulasta is indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with non‑myeloid malignancies receiving myelosuppressive anti‑cancer drugs associated with a clinically significant incidence of febrile neutropenia [see Clinical Studies (14)].
Neulasta is not indicated for the mobilization of peripheral blood progenitor cells for hematopoietic stem cell transplantation.
History
There is currently no drug history available for this drug.
Other Information
Neulasta (pegfilgrastim) is a covalent conjugate of recombinant methionyl human G‑CSF (filgrastim) and monomethoxypolyethylene glycol. Filgrastim is a water‑soluble 175 amino acid protein with a molecular weight of approximately 19 kilodaltons (kD). Filgrastim is obtained from the bacterial fermentation of a strain of E coli transformed with a genetically engineered plasmid containing the human G‑CSF gene. To produce pegfilgrastim, a 20 kD monomethoxypolyethylene glycol molecule is covalently bound to the N‑terminal methionyl residue of filgrastim. The average molecular weight of pegfilgrastim is approximately 39 kD.
Neulasta comes in two presentations:
-
Neulasta for manual subcutaneous injection is supplied in 0.6 mL prefilled syringes.
-
On-body Injector for Neulasta is supplied with a prefilled syringe containing 0.64 mL of Neulasta in solution that delivers 0.6 mL of Neulasta in solution when used with the On-body Injector for Neulasta.
The delivered 0.6 mL dose from either the prefilled syringe for manual subcutaneous injection or the On-body Injector for Neulasta contains 6 mg pegfilgrastim (based on protein weight) in a sterile, clear, colorless, preservative-free solution (pH 4.0) containing acetate (0.35 mg), polysorbate 20 (0.02 mg), sodium (0.02 mg), and sorbitol (30 mg) in Water for Injection, USP.
Sources
Neulasta Manufacturers
-
Amgen Inc
![Neulasta (Pegfilgrastim) Injection [Amgen Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Neulasta | Amgen Inc
![Neulasta (Pegfilgrastim) Injection [Amgen Inc] Neulasta (Pegfilgrastim) Injection [Amgen Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 Recommended DosageThe recommended dosage of Neulasta is a single subcutaneous injection of 6 mg administered once per chemotherapy cycle in adults. Do not administer Neulasta between 14 days before and 24 hours after administration of cytotoxic chemotherapy.
2.2 AdministrationNeulasta is administered subcutaneously via a single prefilled syringe for manual use or for use with the On-body Injector for Neulasta which is co-packaged with a single prefilled syringe.
For manual use or On-body Injector for Neulasta use, visually inspect parenteral drug products (prefilled syringe) for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not administer Neulasta if discoloration or particulates are observed.
The needle cap on the prefilled syringes contains dry natural rubber (derived from latex); persons with latex allergies should not administer these products.
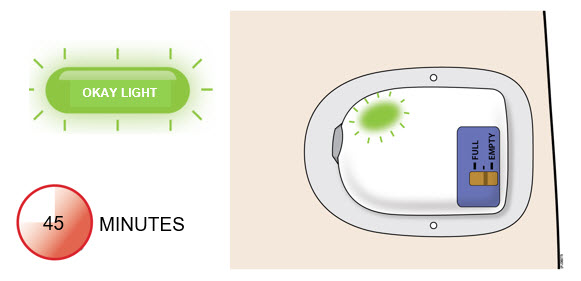
2.3 Special Healthcare Provider Instructions for the On-body Injector for NeulastaA healthcare provider must fill the On-body Injector with Neulasta using the prefilled syringe and then apply the On-body Injector for Neulasta to the patient’s skin (abdomen or back of arm). The back of the arm may only be used if there is a caregiver available to monitor the status of the On-body Injector for Neulasta. Approximately 27 hours after the On-body Injector for Neulasta is applied to the patient’s skin, Neulasta will be delivered over approximately 45 minutes. A healthcare provider may initiate administration with the On-body Injector for Neulasta on the same day as the administration of cytotoxic chemotherapy, as long as the On-body Injector for Neulasta delivers Neulasta no less than 24 hours after administration of cytotoxic chemotherapy.
The prefilled syringe co-packaged in the Neulasta Delivery Kit must only be used with the On-body Injector for Neulasta. The prefilled syringe contains additional solution to compensate for liquid loss during delivery through the On-body Injector for Neulasta. If the prefilled syringe co-packaged in the Neulasta Delivery Kit is used for manual subcutaneous injection, the patient will receive an overdose. If the single use prefilled syringe for manual use is used with the On-body Injector for Neulasta, the patient may receive less than the recommended dose.
Do not use the On-body Injector for Neulasta to deliver any other drug product except the Neulasta prefilled syringe co-packaged with the On-body Injector for Neulasta.
The On-body Injector for Neulasta should be applied to intact, non-irritated skin on the arm or abdomen.
A missed dose could occur due to an On-body Injector for Neulasta failure or leakage. If the patient misses a dose, a new dose should be administered by single prefilled syringe for manual use, as soon as possible after detection.
Refer to the Healthcare Provider Instructions for Use for the On-body Injector for Neulasta for full administration information.
2.4 Advice to Give to Patients Regarding Administration via the On-body Injector for NeulastaAdvise patients to avoid activities such as traveling, driving, or operating heavy machinery during hours 26-29 following application of the On-body Injector for Neulasta (this includes the 45-minute delivery period plus an hour post-delivery). Patients should have a caregiver nearby for the first use.
Refer the patient to the dose delivery information written on the Patient Instructions for Use. Provide training to patients to ensure they understand when the dose delivery of Neulasta will begin and how to monitor the On-body Injector for Neulasta for completed delivery. Ensure patients understand how to identify signs of malfunction of On-body Injector for Neulasta. [see Warnings and Precautions (5.3) and Patient Counseling Information (17)].
Login To Your Free Account