FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
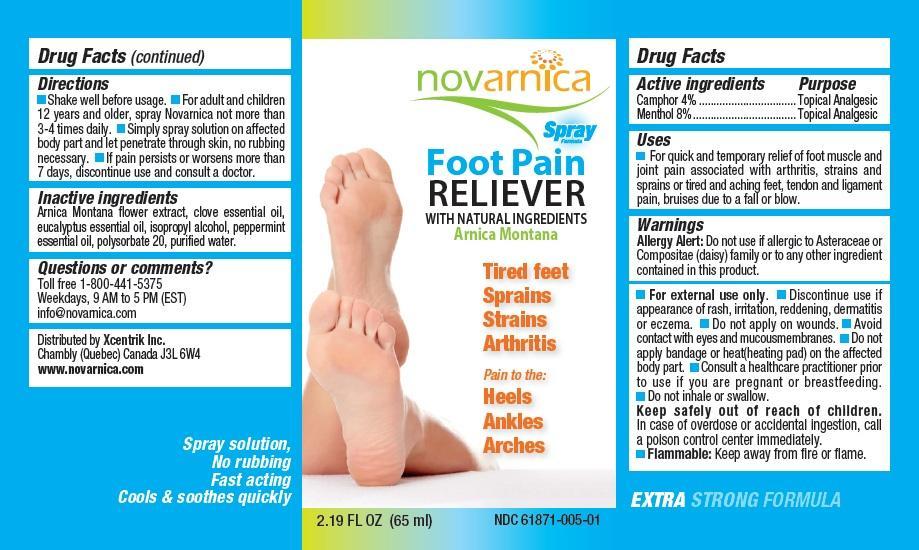
Novarnica Foot Pain Reliever Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Allergy Alert: Do not use if allergic to Asteraceae or Compositae (daisy) family or to any other ingredient contained in this product.
- For external use only.
- Discontinue use if appearance of rash, irritation, reddening, dermatitis or eczema.
- Do not apply on wounds.
- Avoid contact with eyes and mucous membranes.
- Do not apply bandage or heat (heating pad) on the affected body part.
- Consult a healthcare practitioner prior to use if you are pregnant or breastfeeding.
- Do not inhale or swallow.
- Keep safely out of reach of children. In case of overdose or accidental ingestion, call a poison control center immediately.
- Flammable: Keep away from fire or flame.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
For quick and temporary relief of foot muscle and joint pain associated with arthritis, strains and sprains or tired and aching feet, tendon and ligament pain, bruises due to a fall or blow.
History
There is currently no drug history available for this drug.
Other Information
There are no additional details available for this product.
Sources
Novarnica Foot Pain Reliever Manufacturers
-
Xcentrik Inc.
Login To Your Free Account