FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Otomax Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
The use of OTOMAX ointment has been associated with deafness or partial hearing loss in a small number of sensitive dogs (eg, geriatric). The hearing deficit is usually temporary. If hearing or vestibular dysfunction is noted during the course of treatment, discontinue use of OTOMAX ointment immediately and flush the ear canal thoroughly with a nonototoxic solution.
Corticosteroids administered to dogs, rabbits, and rodents during pregnancy have resulted in cleft palate in offspring. Other congenital anomalies including deformed forelegs, phocomelia, and anasarca have been reported in offspring of dogs which received corticosteroids during pregnancy.
Clinical and experimental data have demonstrated that corticosteroids administered orally or parenterally to animals may induce the first stage of parturition if used during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta, and metritis.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
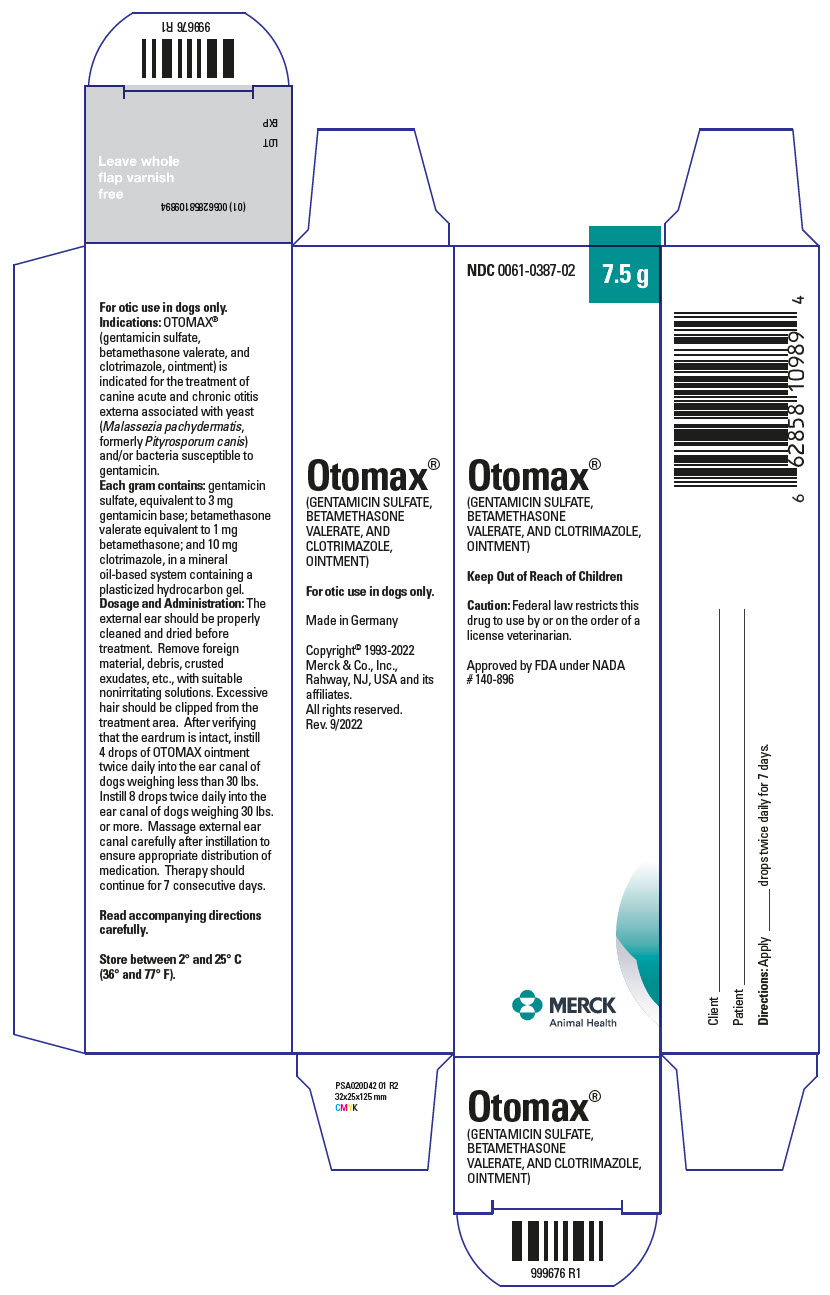
OTOMAX ointment is indicated for the treatment of canine acute and chronic otitis externa associated with yeast (Malassezia pachydermatis, formerly Pityrosporum canis) and/or bacteria susceptible to gentamicin.
History
There is currently no drug history available for this drug.
Other Information
Each gram of OTOMAX ointment contains gentamicin sulfate, USP equivalent to 3 mg gentamicin base; betamethasone valerate, USP equivalent to 1 mg betamethasone; and 10 mg clotrimazole, USP in a mineral oil-based system containing a plasticized hydrocarbon gel.
Sources
Otomax Manufacturers
-
Merck Sharp & Dohme Corp.
![Otomax (Gentamicin Sulfate, Betamethasone Valerate, And Clotrimazole) Ointment [Merck Sharp & Dohme Corp.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Otomax | Merck Sharp & Dohme Corp.
![Otomax (Gentamicin Sulfate, Betamethasone Valerate, And Clotrimazole) Ointment [Merck Sharp & Dohme Corp.] Otomax (Gentamicin Sulfate, Betamethasone Valerate, And Clotrimazole) Ointment [Merck Sharp & Dohme Corp.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
The external ear should be thoroughly cleaned and dried before treatment. Remove foreign material, debris, crusted exudates, etc., with suitable nonirritating solutions. Excessive hair should be clipped from the treatment area. After verifying that the eardrum is intact, instill 4 drops from the 7.5 g and 15 g tube, and 15 g and 30 g bottle (2 drops from the 215 g bottle) of OTOMAX ointment twice daily into the ear canal of dogs weighing less than 30 lbs. Instill 8 drops from the 7.5 g and 15 g tube, and 15 g and 30 g bottle (4 drops from the 215 g bottle) twice daily into the ear canal of dogs weighing 30 lbs or more. Massage external ear canal carefully after instillation to ensure appropriate distribution of medication. Therapy should continue for 7 consecutive days.
Login To Your Free Account