Skelaxin Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
SKELAXIN may enhance the effects of alcohol and other CNS depressants.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
SKELAXIN (metaxalone) is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified, but may be related to its sedative properties. Metaxalone does not directly relax tense skeletal muscles in man.
History
There is currently no drug history available for this drug.
Other Information
SKELAXIN ® (metaxalone) is available as an 800 mg oval, scored pink tablet.
Chemically, metaxalone is 5-[(3,5- dimethylphenoxy) methyl]-2-oxazolidinone. The empirical formula is C 12H 15NO 3, which corresponds to a molecular weight of 221.25. The structural formula is:
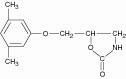
Metaxalone is a white to almost white, odorless crystalline powder freely soluble in chloroform, soluble in methanol and in 96% ethanol, but practically insoluble in ether or water.
Each tablet contains 800 mg metaxalone and the following inactive ingredients: alginic acid, ammonium calcium alginate, B-Rose Liquid, corn starch, and magnesium stearate.
Sources

_KING(60793-136-05)_REV1.jpg)

![Skelaxin (Metaxalone) Tablet [Physicians Total Care, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=cb28abf4-608f-4dcb-b179-9dcf71274dbb&name=packagelabel.jpg)
![Skelaxin (Metaxalone) Tablet [Rebel Distributors Corp]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=cb47d76e-a7e3-4bac-8074-8d7e95c6a6eb&name=cb47d76e-a7e3-4bac-8074-8d7e95c6a6eb-03.jpg)
![Skelaxin (Metaxalone) Tablet [Preferred Pharmaceuticals, Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d8684144-6c2d-4664-943a-2d3d2b46b1fc&name=metalaxone.jpg)
![Skelaxin (Metaxalone) Tablet [Cardinal Health]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=24da58e8-86e8-4206-9ad3-4ebb27522cc8&name=bc98621b-1cd5-4ee8-9f15-f90946dd39a9-03.jpg)
![Skelaxin (Metaxalone) Tablet [Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0485d087-a215-4f61-8687-4161b9a55f06&name=skelaxin800mgcorepharma.jpg)
![Skelaxin (Metaxalone) Tablet [Stat Rx Usa Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=5e1ec092-4748-47c6-9b72-1b826da0ce02&name=SKELAXIN800MGLABEL.jpg)
![Skelaxin (Metaxalone) Tablet [Pd-rx Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=eca7792e-c07b-4a42-9b2b-48a4366b77a6&name=55289736.jpg)
![Skelaxin (Metaxalone) Tablet [Pfizer Laboratories Div Pfizer Inc]](https://www.recallguide.org/wp-content/themes/bootstrap/assets/img/drug-image-placeholder.jpg)