FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Sominex Max Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
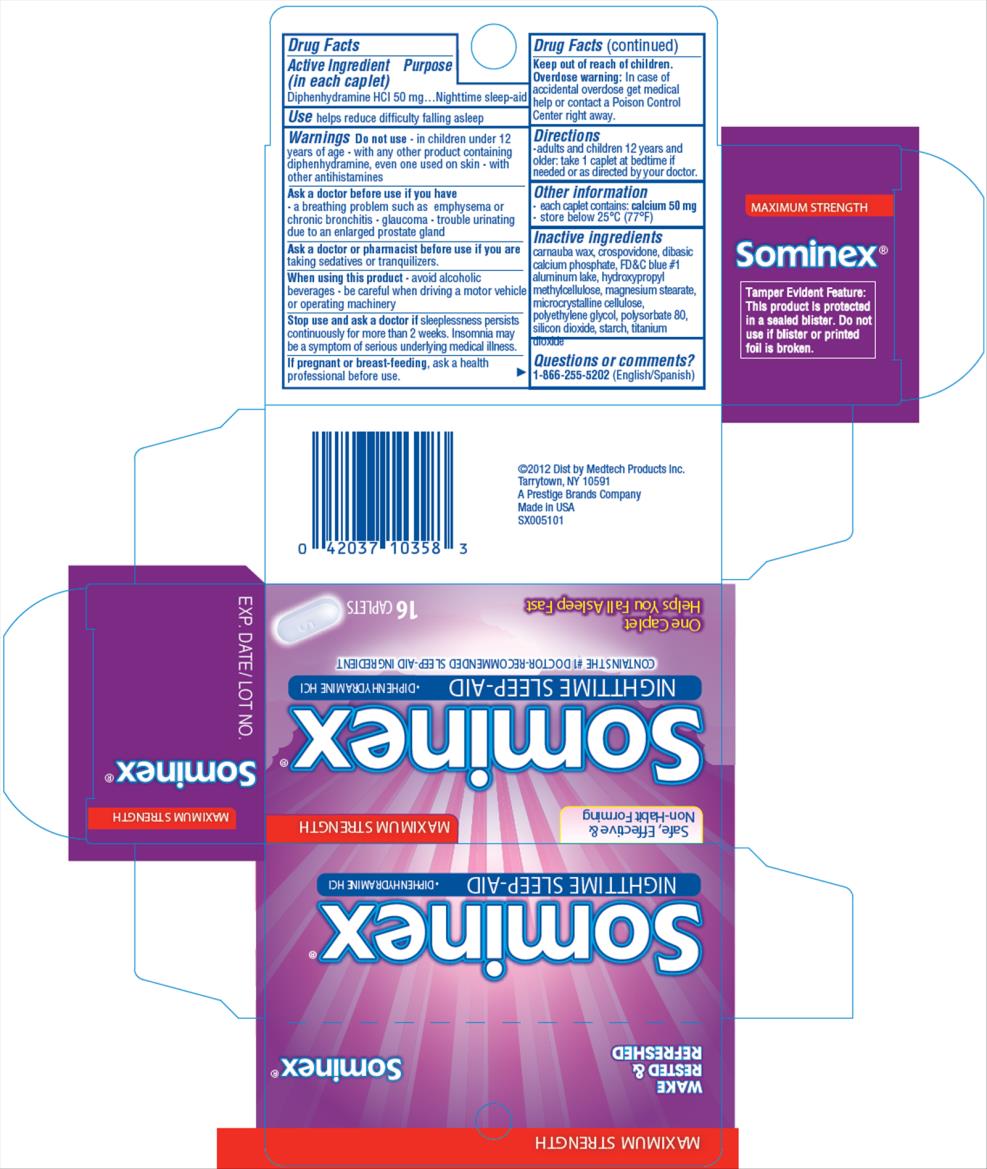
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- with other antihistamines
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
taking sedatives or tranquilizers
avoid alcoholic beverages
- be careful when driving a motor vehicle or operating machinery
sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
ask a health professional before use.
Overdose warning: In case of accidental overdose get medical help or contact a Poison Control Center right away.
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- with other antihistamines
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
taking sedatives or tranquilizers
Stop use and ask a doctor ifsleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
helps reduce difficultly falling asleep
History
There is currently no drug history available for this drug.
Other Information
There are no additional details available for this product.
Sources
Sominex Max Manufacturers
-
Medtech Products Inc.
Login To Your Free Account