Ssd Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is potential cross-sensitivity between silver sulfadiazine and other sulfonamides. If allergic reactions attributable to treatment with silver sulfadiazine occur, continuation of therapy must be weighed against the potential hazards of the particular allergic reaction.
Fungal proliferation in and below the eschar may occur. However, the incidence of clinically reported fungal superinfection is low.
The use of Silver Sulfadiazine Cream in some cases of glucose-6-phosphate dehydrogenase-deficient individuals may be hazardous, as hemolysis may occur.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Silver Sulfadiazine Cream is a topical antimicrobial drug indicated as an adjunct for the prevention and treatment of wound sepsis in patients with second and third degree burns.
History
There is currently no drug history available for this drug.
Other Information
SSD (1% Silver Sulfadiazine) Cream and SSD AF (1% Silver Sulfadiazine) Cream are topical antibacterial preparations which have as their active antimicrobial ingredient silver sulfadiazine. The active moiety is contained within an opaque, white, water miscible cream base.
Each 1000 grams of SSD/SSD AF Cream contains 10 grams of silver sulfadiazine
Inactive Ingredients: cetyl alcohol (SSD Cream only), isopropyl myristate, polyoxyl 40 stearate, propylene glycol, purified water, stearyl alcohol, sodium hydroxide, sorbitan monooleate, white petrolatum; with 0.3% methyl paraben, as a preservative.
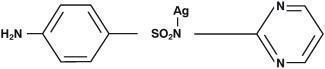
Silver sulfadiazine has an emprical formula of C10H9AgN4O2S, molecular weight of 357.14 and structural formula as shown:
Sources




![Ssd Cream (Silver Sulfadiazine) Cream [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=78b8a01e-170a-4160-a221-9d2dc64e22f7&name=MM2.jpg)
![Ssd Cream (Silver Sulfadiazine) Cream [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=4479bbda-b1d7-4746-8c1f-17e0a6abe7fa&name=MM2.jpg)
![Ssd Cream (Silver Sulfadiazine) Cream [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=7b81290e-b0c2-4908-8373-6a64c6b4de6f&name=MM2.jpg)
![Ssd (Silver Sulfadiazine) Cream [Par Pharmaceutical Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=b8273952-73c4-4bdb-bee2-7a712ace321b&name=d47a0bcf-60c2-4e3d-b1a7-6eb02e165591-02.jpg)
![Ssd Cream (Silver Sulfadiazine) Cream [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e44599fe-d6d1-4667-bd3a-a9e7609187ea&name=MM2.jpg)
![Ssd Cream (Silver Sulfadiazine) Cream [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=cecc0b75-fccf-418d-923a-2a5869c1d043&name=MM2.jpg)
![Ssd Cream (Silver Sulfadiazine) Cream [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=a7defb53-adcc-4740-a112-46e3e367af12&name=MM2.jpg)
![Ssd Cream (Silver Sulfadiazine) Cream [Dr Reddys Laboratories Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=30d6dc33-e457-466b-9159-d8e7cd791820&name=25g-tube-cont-label.jpg)