Tazorac Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Pregnancy Category X. See CONTRAINDICATIONS section. Women of child-bearing potential should be warned of the potential risk and use adequate birth-control measures when TAZORAC® Gel is used. The possibility that a woman of childbearing potential is pregnant at the time of institution of therapy should be considered. A negative result for pregnancy test having a sensitivity down to at least 50 mIU/mL for hCG should be obtained within 2 weeks prior to TAZORAC® Gel therapy, which should begin during a normal menstrual period.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
TAZORAC® (tazarotene) Gel 0.05% and 0.1% are indicated for the topical treatment of patients with stable plaque psoriasis of up to 20% body surface area involvement.
TAZORAC® (tazarotene) Gel 0.1% is also indicated for the topical treatment of patients with facial acne vulgaris of mild to moderate severity.
The efficacy of TAZORAC® Gel in the treatment of acne previously treated with other retinoids or resistant to oral antibiotics has not been established.
History
There is currently no drug history available for this drug.
Other Information
TAZORAC® Gel is a translucent, aqueous gel and contains the compound tazarotene, a member of the acetylenic class of retinoids. It is for topical dermatologic use only. The active ingredient is represented by the following structural formula:
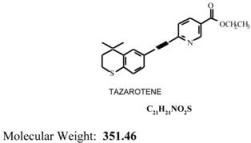
Chemical Name:
Ethyl 6-[2-(4,4-dimethylthiochroman-6-yl) ethynyl] nicotinate
Contains:
Active: Tazarotene................................... 0.05% or 0.1% (w/w)
Preservative: Benzyl alcohol.................................. 1.0% (w/w)
Inactives: Ascorbic acid, butylated hydroxyanisole, butylated hydroxytoluene, carbomer 934P, edetate disodium, hexylene glycol, poloxamer 407, polyethylene glycol 400, polysorbate 40, purified water, and tromethamine.
Sources