FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Xolair Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Xolair is indicated for adults and adolescents (12 years of age and above) with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and whose symptoms are inadequately controlled with inhaled corticosteroids.
Xolair has been shown to decrease the incidence of asthma exacerbations in these patients.
Limitations of Use:
- Xolair is not indicated for the relief of acute bronchospasm or status asthmaticus.
- Xolair is not indicated for treatment of other allergic conditions.
Xolair is indicated for the treatment of adults and adolescents (12 years of age and above) with chronic idiopathic urticaria who remain symptomatic despite H1 antihistamine treatment.
Limitation of Use:
Xolair is not indicated for treatment of other forms of urticaria.
History
There is currently no drug history available for this drug.
Other Information
Xolair is a recombinant DNA-derived humanized IgG1κ monoclonal antibody that selectively binds to human immunoglobulin E (IgE). The antibody has a molecular weight of approximately 149 kiloDaltons. Xolair is produced by a Chinese hamster ovary cell suspension culture in a nutrient medium containing the antibiotic gentamicin. Gentamicin is not detectable in the final product.
Xolair is a sterile, white, preservative free, lyophilized powder contained in a single use vial that is reconstituted with Sterile Water for Injection (SWFI), USP, and administered as a subcutaneous (SC) injection. Each 202.5 mg vial of omalizumab also contains L-histidine (1.8 mg), L-histidine hydrochloride monohydrate (2.8 mg), polysorbate 20 (0.5 mg) and sucrose (145.5 mg) and is designed to deliver 150 mg of omalizumab in 1.2 mL after reconstitution with 1.4 mL SWFI, USP.
Sources
Xolair Manufacturers
-
Genentech, Inc.
![Xolair (Omalizumab) Injection, Solution [Genentech, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Xolair | Genentech, Inc.
![Xolair (Omalizumab) Injection, Solution [Genentech, Inc.] Xolair (Omalizumab) Injection, Solution [Genentech, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
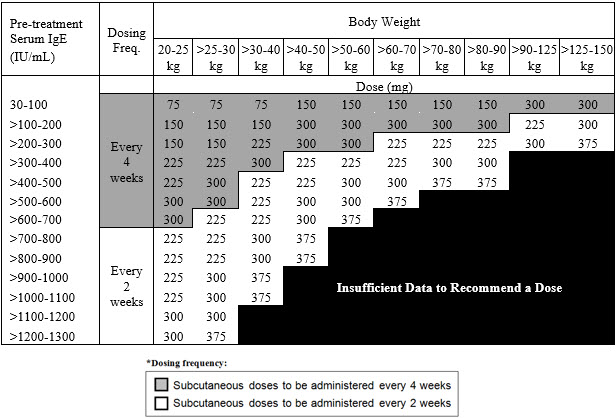
2.1 Dosage for AsthmaAdminister Xolair 150 to 375 mg by subcutaneous injection every 2 or 4 weeks. Determine doses (mg) and dosing frequency by serum total IgE level (IU/mL), measured before the start of treatment, and body weight (kg) (see Table 1 and 2).
Adjust doses for significant changes in body weight (see Table 1 and 2).
Total IgE levels are elevated during treatment and remain elevated for up to one year after the discontinuation of treatment. Therefore, re-testing of IgE levels during Xolair treatment cannot be used as a guide for dose determination.
Interruptions lasting less than one year: Dose based on serum IgE levels obtained at the initial dose determination. Interruptions lasting one year or more: Re-test total serum IgE levels for dose determination.Periodically reassess the need for continued therapy based upon the patient's disease severity and level of asthma control.
Table 1. Subcutaneous Xolair Doses Every 4 Weeks for Patients 12 Years of Age and Older with Asthma Pre-treatment Serum IgE Body Weight 30–60 kg > 60–70 kg > 70–90 kg > 90–150 kg ≥ 30–100 IU/mL 150 mg 150 mg 150 mg 300 mg > 100–200 IU/mL 300 mg 300 mg 300 mg > 200–300 IU/mL 300 mg > 300–400 IU/mL SEE TABLE 2 > 400–500 IU/mL > 500–600 IU/mL Table 2. Subcutaneous Xolair Doses Every 2 Weeks for Patients 12 Years of Age and Older with Asthma Pre-treatment Serum IgE Body Weight 30–60 kg > 60–70 kg > 70–90 kg > 90–150 kg ≥ 30–100 IU/mL SEE TABLE 1 > 100–200 IU/mL 225 mg > 200–300 IU/mL 225 mg 225 mg 300 mg > 300–400 IU/mL 225 mg 225 mg 300 mg > 400–500 IU/mL 300 mg 300 mg 375mg > 500–600 IU/mL 300 mg 375 mg DO NOT DOSE > 600–700 IU/mL 375 mg 2.2 Dosage for Chronic Idiopathic UrticariaAdminister Xolair 150 or 300 mg by subcutaneous injection every 4 weeks.
Dosing of Xolair in CIU patients is not dependent on serum IgE (free or total) level or body weight.
The appropriate duration of therapy for CIU has not been evaluated. Periodically reassess the need for continued therapy.
2.3 ReconstitutionThe supplied Xolair lyophilized powder must be reconstituted with Sterile Water for Injection (SWFI) USP, using the following instructions:
1) Before reconstitution, determine the number of vials that will need to be reconstituted (each vial delivers 150 mg of Xolair) [see Dosage and Administration (2.1, 2.2)]. 2) Draw 1.4 mL of SWFI, USP, into a 3 mL syringe equipped with a 1 inch, 18-gauge needle. 3) Place the vial upright on a flat surface and using standard aseptic technique, insert the needle and inject the SWFI, USP, directly onto the product. 4) Keeping the vial upright, gently swirl the upright vial for approximately 1 minute to evenly wet the powder. Do not shake. 5) Gently swirl the vial for 5 to 10 seconds approximately every 5 minutes in order to dissolve any remaining solids. The lyophilized product takes 15 to 20 minutes to dissolve. If it takes longer than 20 minutes to dissolve completely, gently swirl the vial for 5 to 10 seconds approximately every 5 minutes until there are no visible gel-like particles in the solution. Do not use if the contents of the vial do not dissolve completely by 40 minutes. 6) After reconstitution, Xolair solution is somewhat viscous and will appear clear or slightly opalescent. It is acceptable if there are a few small bubbles or foam around the edge of the vial; there should be no visible gel-like particles in the reconstituted solution. Do not use if foreign particles are present. 7) Invert the vial for 15 seconds in order to allow the solution to drain toward the stopper. 8) Use the Xolair solution within 8 hours following reconstitution when stored in the vial at 2 to 8ºC (36 to 46ºF), or within 4 hours of reconstitution when stored at room temperature. Reconstituted Xolair vials should be protected from sunlight. 9) Using a new 3 mL syringe equipped with a 1-inch, 18-gauge needle, insert the needle into the inverted vial. Position the needle tip at the very bottom of the solution in the vial stopper when drawing the solution into the syringe. The reconstituted product is somewhat viscous; in order to obtain the full 1.2 mL dose, all of the product must be withdrawn from the vial before expelling any air or excess solution from the syringe. Before removing the needle from the vial, pull the plunger all the way back to the end of the syringe barrel in order to remove all of the solution from the inverted vial. 10) Replace the 18-gauge needle with a 25-gauge needle for subcutaneous injection. 11) Expel air, large bubbles, and any excess solution in order to obtain the required 1.2 mL dose. A thin layer of small bubbles may remain at the top of the solution in the syringe. 2.4 AdministrationAdminister Xolair by subcutaneous injection. The injection may take 5-10 seconds to administer because the solution is slightly viscous. Do not administer more than 150 mg (contents of one vial) per injection site. Divide doses of more than 150 mg among two or more injection sites (Table 3).
Table 3. Number of Injections and Total Injection Volumes Xolair Dose* Number of Injections Total Volume Injected * All doses in the table are approved for use in asthma patients. The 150 mg and 300 mg Xolair doses are intended for use in CIU patients. 150 mg 1 1.2 mL 225 mg 2 1.8 mL 300 mg 2 2.4 mL 375mg 3 3.0 mL
Login To Your Free Account