FDA records indicate that there are no current recalls for this drug.
Zoloft (sertraline hydrochloride)
Zoloft® is an antidepressant medication classified as a selective serotonin reuptake inhibitor (SSRI), generally prescribed to adults to treat major depression and anxiety disorders and, less commonly, for vascular headaches and premature ejaculation. It is an oral medication originally brought to the market by American pharmaceutical manufacturer Pfizer and is available in 25mg, 50mg, and 100mg capsules.
Are you a medical professional?
Trending Topics
Zoloft Recall
Get an alert when a recall is issued.
Questions & Answers
Question
Zoloft makes me more moody and more depressed. I didn’t take it for long.
Side Effects & Adverse Reactions
Common side effects include fatigue, insomnia, nausea, dry mouth, headache, diarrhea, dizziness, weight gain, and sexual dysfunction (difficulty achieving arousal, erection and/or orgasm).
As with all antidepressants, Zoloft carries the FDA’s black box warning due to an increased risk of suicidal thoughts/ideation and actions, especially in children and young adults. (fda.gov)
The use of Zoloft during the first trimester of pregnancy is associated with increased risks of the following birth defects: omphalocele (abdominal wall defects), anal atresia (malformation of the anus/rectum), limb reduction, and septal (heart) defects.(ncbi.nlm.nih.gov)
Like many other antidepressant medications, the discontinuation of regular use of Zoloft can cause numerous unpleasant withdrawal side effects, including flu-like symptoms (nausea, vomiting, sweating, headaches, diarrhea), sleep disturbances (insomnia, nightmares, fatigue), sensory/movement disturbances (vertigo, dizziness, “zap” like electrical sensations in the brain or nerve paths), and mood disturbances (anxiety, dysphoria, agitation). Most cases of these withdrawal symptoms are mild and resolve themselves after 1-4 weeks.
Possible Adverse Interactions
Zoloft should not be taken with, or immediately after stopping use of, monoamine oxidase inhibitors (MAOIs), another class of commonly used antidepressants, as this can cause serotonin syndrome or serotonin toxicity, a condition that can be fatal.
It is also contraindicated for use with the antipsychotic medication pimozide (Orap) due to the risk of serious heart problems. The concentrated form also contains an alcohol solution, so it is recommended not to take it with Antabuse, a medication used to treat alcoholism. Grapefruit and grapefruit juice should also be avoided, as it can increase the levels of Zoloft in the body.
Legal Issues
Pfizer has over 1,000 lawsuits pending against them regarding the use of Zoloft causing birth defects in pregnancy. In June 2015, a jury in Philadelphia, PA sided with the defendant (Pfizer) in a case wherein the plaintiffs alleged that Pfizer did not sufficiently warn patients of the potential for birth defects, finding that the drug’s label adequately warned physicians to weigh and discuss the benefits and risks of use before prescribing it (Robinson v. Wolters Kluwer Health Inc. and Pfizer, Inc.). This is the second such judgment in favor of Pfizer regarding birth defects and use of Zoloft in as many trials. (bloomberg.com)
Zoloft was also the subject of a consumer fraud class action lawsuit that was filed in 2013 in California. The case, Plumlee vs. Pfizer Inc., centered on claims by plaintiff Laura Plumlee that she had taken the drug as prescribed for 3 years with absolutely no effect. The suit alleged that Pfizer deliberately omitted from labeling that there had been studies conducted that found Zoloft to be no more effective than a placebo, and that their marketing and advertising regarding its effectiveness was misleading. The plaintiff’s first claim was dismissed in March 2014, not due to the argument of effectiveness or lack thereof, but due to time-barring. Plumlee had last used Zoloft in 2008, but did not file the lawsuit until 2013. She was given the opportunity to amend her claim and re-file, but the suit was ultimately dismissed with prejudice in September 2014.
FDA Safety Alerts
July, 2006
Food and Drug Administration Labeling Changes indicate several changes but this specific Alert was issued warning of increased risk of Neonatal Persistent Pulmonary Hypertension in mothers taking the drug (fda.gov).
December 14, 2011
The FDA warns of pregnancy risks in SSRI’s in general, including Zoloft. (fda.gov).
July 26, 2011
FDA warns of serious CNS reactions in patients taking the antibiotic linezolid (Zyvox) and certain psychiatric serotonin drugs, including Zoloft. (fda.gov)
Manufacturer Warnings
An undated warning letter from Pfizer alerts healthcare providers to the warnings and label changes regarding detrimental interactions of Zoloft with pimozide (Orap) and MAOIs (fda.gov).
FDA Labeling Changes
September 2014
A labeling adjustment was made to the drug interactions to remove previous additions of QT prolongation, Torsades de Pointes, and ventricular tachycardia – all types of cardiac arrhythmia (irregular heart rhythm) (fda.gov).
February 2013
Diabetes mellitus (scientific name for both Type 1 and Type 2 diabetes) was added to the list of events observed during the post-marketing evaluation of Zoloft. (fda.gov)
December 2012
Contraindications and warnings were added regarding the types of drugs or substances that will interact with use of Zoloft and possibly cause serotonin syndrome, expanding upon the warnings issued in January 2009 (fda.gov).
August 2011
Information was added to the packaging label to advise caution when co-administering Zoloft with drugs that may enhance serotonergic neurotransmission (transmission/absorption of serotonin) due to possibility for interaction. In addition, a precaution was added regarding laboratory testing: patients using Zoloft were reported to have received false positives on urine immunoassay screening tests for benzodiazepines(a class of frequently abused anti-anxiety medications) such as clonazepam (fda.gov).
January 2009
A warning was issued regarding the potential for the potentially fatal complication serotonin syndrome, also known as serotonin storm or serotonin toxicity, or a similar condition called neuroleptic malignant syndrome. Both of these conditions can occur when Zoloft is taken in combination with MAOIs (such as selegiline or phenelzine), or other drugs that alter the neurotransmission of serotonin (fda.gov).
March 2008
Precautions were added to the label making changes to the following subjects: abnormal bleeding as a potential side effect, and interactions with drugs that interfere with the clotting and flow of blood (Non-selective NSAIDs, aspirin, warfarin, etc.) (fda.gov).
Uses
Zoloft® is an antidepressant medication classified as a selective serotonin reuptake inhibitor (SSRI), generally prescribed to adults to treat major depression and anxiety disorders and, less commonly, for vascular headaches and premature ejaculation. It is an oral medication originally brought to the market by American pharmaceutical manufacturer Pfizer and is available in 25mg, 50mg, and 100mg capsules.
History
Sertraline was the result of many levels of experimentation by Pfizer chemists, beginning in the early 1970’s with the invention of multiple psychoactive compounds that were based on the structures of some of the earliest available antipsychotic medications. These experiments would eventually result in the development of many neurotransmitter reuptake inhibitors, the most potent and selective of which would go on to be tested in vivo (on living organisms, including human trials) and dubbed sertraline. None of the scientists responsible for its creation were anticipating the production of an SSRI type of antidepressant, so the discovery is considered a happy accident.
Zoloft was approved by the FDA on the recommendation of the Psychopharmacological Drugs Advisory Committee in 1991. It was also made available in the United Kingdom the previous year, and in Australia in 1994. It was only approved for prescription to adults over the age of 18 until 2002, when the FDA approved its use in children over age 6 to treat severe cases of obsessive-compulsive disorder. In 2005, the FDA issued a black box warning for all antidepressants, including sertraline, concerning suicidal thoughts and behaviors in children; this warning was amended in 2007 to include the possibility of suicidal tendencies in young adults aged 18 to 24.
The patent for Zoloft expired in the US in 2006, allowing for the availability of sertraline in its generic form. Other brand names for sertraline include Lustral and Asentra. In 2013, it was the most prescribed antidepressant and second-most prescribed psychiatric medication in the United States. (psychentral.com)
Other Information
How It Works
As an SSRI, Zoloft is thought to work by inhibiting the passage of serotonin between two nerve cells, allowing for the serotonin to stay in the synapse (gap) between the cells, thereby increasing its availability to bind with the proteins in the receiving cell. This increase in available serotonin is believed to positively affect mood and manage depression.
Sources
Zoloft Manufacturers
-
Stat Rx Usa Llc
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Stat Rx Usa Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Zoloft | Stat Rx Usa Llc
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Stat Rx Usa Llc] Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Stat Rx Usa Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Initial Treatment Dosage for Adults Major Depressive Disorder and Obsessive-Compulsive DisorderZOLOFT treatment should be administered at a dose of 50 mg once daily.
Panic Disorder, Posttraumatic Stress Disorder and Social Anxiety DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily. After one week, the dose should be increased to 50 mg once daily.
While a relationship between dose and effect has not been established for major depressive disorder, OCD, panic disorder, PTSD or social anxiety disorder, patients were dosed in a range of 50–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for the treatment of these indications. Consequently, a dose of 50 mg, administered once daily, is recommended as the initial therapeutic dose. Patients not responding to a 50 mg dose may benefit from dose increases up to a maximum of 200 mg/day. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
Premenstrual Dysphoric DisorderZOLOFT treatment should be initiated with a dose of 50 mg/day, either daily throughout the menstrual cycle or limited to the luteal phase of the menstrual cycle, depending on physician assessment.
While a relationship between dose and effect has not been established for PMDD, patients were dosed in the range of 50–150 mg/day with dose increases at the onset of each new menstrual cycle (see Clinical Trials under CLINICAL PHARMACOLOGY). Patients not responding to a 50 mg/day dose may benefit from dose increases (at 50 mg increments/menstrual cycle) up to 150 mg/day when dosing daily throughout the menstrual cycle, or 100 mg/day when dosing during the luteal phase of the menstrual cycle. If a 100 mg/day dose has been established with luteal phase dosing, a 50 mg/day titration step for three days should be utilized at the beginning of each luteal phase dosing period.
ZOLOFT should be administered once daily, either in the morning or evening.
Dosage for Pediatric Population (Children and Adolescents) Obsessive-Compulsive DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily in children (ages 6–12) and at a dose of 50 mg once daily in adolescents (ages 13–17).
While a relationship between dose and effect has not been established for OCD, patients were dosed in a range of 25–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for pediatric patients (6–17 years) with OCD. Patients not responding to an initial dose of 25 or 50 mg/day may benefit from dose increases up to a maximum of 200 mg/day. For children with OCD, their generally lower body weights compared to adults should be taken into consideration in advancing the dose, in order to avoid excess dosing. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
ZOLOFT should be administered once daily, either in the morning or evening.
Maintenance/Continuation/Extended Treatment Major Depressive DisorderIt is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy beyond response to the acute episode. Systematic evaluation of ZOLOFT has demonstrated that its antidepressant efficacy is maintained for periods of up to 44 weeks following 8 weeks of initial treatment at a dose of 50–200 mg/day (mean dose of 70 mg/day) (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Posttraumatic Stress DisorderIt is generally agreed that PTSD requires several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in PTSD is maintained for periods of up to 28 weeks following 24 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Social Anxiety DisorderSocial anxiety disorder is a chronic condition that may require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in social anxiety disorder is maintained for periods of up to 24 weeks following 20 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). Dosage adjustments should be made to maintain patients on the lowest effective dose and patients should be periodically reassessed to determine the need for long-term treatment.
Obsessive-Compulsive Disorder and Panic DisorderIt is generally agreed that OCD and Panic Disorder require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of continuing ZOLOFT for periods of up to 28 weeks in patients with OCD and Panic Disorder who have responded while taking ZOLOFT during initial treatment phases of 24 to 52 weeks of treatment at a dose range of 50–200 mg/day has demonstrated a benefit of such maintenance treatment (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Nevertheless, patients should be periodically reassessed to determine the need for maintenance treatment.
Premenstrual Dysphoric DisorderThe effectiveness of ZOLOFT in long-term use, that is, for more than 3 menstrual cycles, has not been systematically evaluated in controlled trials. However, as women commonly report that symptoms worsen with age until relieved by the onset of menopause, it is reasonable to consider continuation of a responding patient. Dosage adjustments, which may include changes between dosage regimens (e.g., daily throughout the menstrual cycle versus during the luteal phase of the menstrual cycle), may be needed to maintain the patient on the lowest effective dosage and patients should be periodically reassessed to determine the need for continued treatment.
Switching Patients to or from a Monoamine Oxidase InhibitorAt least 14 days should elapse between discontinuation of an MAOI and initiation of therapy with ZOLOFT. In addition, at least 14 days should be allowed after stopping ZOLOFT before starting an MAOI (see CONTRAINDICATIONS and WARNINGS).
Special Populations Dosage for Hepatically Impaired PatientsThe use of sertraline in patients with liver disease should be approached with caution. The effects of sertraline in patients with moderate and severe hepatic impairment have not been studied. If sertraline is administered to patients with liver impairment, a lower or less frequent dose should be used (see CLINICAL PHARMACOLOGY and PRECAUTIONS).
Treatment of Pregnant Women During the Third TrimesterNeonates exposed to ZOLOFT and other SSRIs or SNRIs, late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding (see PRECAUTIONS). When treating pregnant women with ZOLOFT during the third trimester, the physician should carefully consider the potential risks and benefits of treatment. The physician may consider tapering ZOLOFT in the third trimester.
Discontinuation of Treatment with ZoloftSymptoms associated with discontinuation of ZOLOFT and other SSRIs and SNRIs, have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
ZOLOFT Oral ConcentrateZOLOFT Oral Concentrate contains 20 mg/mL of sertraline (as the hydrochloride) as the active ingredient and 12% alcohol. ZOLOFT Oral Concentrate must be diluted before use. Just before taking, use the dropper provided to remove the required amount of ZOLOFT Oral Concentrate and mix with 4 oz (1/2 cup) of water, ginger ale, lemon/lime soda, lemonade or orange juice ONLY. Do not mix ZOLOFT Oral Concentrate with anything other than the liquids listed. The dose should be taken immediately after mixing. Do not mix in advance. At times, a slight haze may appear after mixing; this is normal. Note that caution should be exercised for patients with latex sensitivity, as the dropper dispenser contains dry natural rubber.
ZOLOFT Oral Concentrate is contraindicated with ANTABUSE (disulfiram) due to the alcohol content of the concentrate.
-
Pd-rx Pharmaceuticals, Inc.
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Pd-rx Pharmaceuticals, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Zoloft | Pd-rx Pharmaceuticals, Inc.
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Pd-rx Pharmaceuticals, Inc.] Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Pd-rx Pharmaceuticals, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Initial Treatment Dosage for Adults Major Depressive Disorder and Obsessive-Compulsive DisorderZOLOFT treatment should be administered at a dose of 50 mg once daily.
Panic Disorder, Posttraumatic Stress Disorder and Social Anxiety DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily. After one week, the dose should be increased to 50 mg once daily.
While a relationship between dose and effect has not been established for major depressive disorder, OCD, panic disorder, PTSD or social anxiety disorder, patients were dosed in a range of 50–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for the treatment of these indications. Consequently, a dose of 50 mg, administered once daily, is recommended as the initial therapeutic dose. Patients not responding to a 50 mg dose may benefit from dose increases up to a maximum of 200 mg/day. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
Premenstrual Dysphoric DisorderZOLOFT treatment should be initiated with a dose of 50 mg/day, either daily throughout the menstrual cycle or limited to the luteal phase of the menstrual cycle, depending on physician assessment.
While a relationship between dose and effect has not been established for PMDD, patients were dosed in the range of 50–150 mg/day with dose increases at the onset of each new menstrual cycle (see Clinical Trials under CLINICAL PHARMACOLOGY). Patients not responding to a 50 mg/day dose may benefit from dose increases (at 50 mg increments/menstrual cycle) up to 150 mg/day when dosing daily throughout the menstrual cycle, or 100 mg/day when dosing during the luteal phase of the menstrual cycle. If a 100 mg/day dose has been established with luteal phase dosing, a 50 mg/day titration step for three days should be utilized at the beginning of each luteal phase dosing period.
ZOLOFT should be administered once daily, either in the morning or evening.
Dosage for Pediatric Population (Children and Adolescents) Obsessive-Compulsive DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily in children (ages 6–12) and at a dose of 50 mg once daily in adolescents (ages 13–17).
While a relationship between dose and effect has not been established for OCD, patients were dosed in a range of 25–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for pediatric patients (6–17 years) with OCD. Patients not responding to an initial dose of 25 or 50 mg/day may benefit from dose increases up to a maximum of 200 mg/day. For children with OCD, their generally lower body weights compared to adults should be taken into consideration in advancing the dose, in order to avoid excess dosing. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
ZOLOFT should be administered once daily, either in the morning or evening.
Maintenance/Continuation/Extended Treatment Major Depressive DisorderIt is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy beyond response to the acute episode. Systematic evaluation of ZOLOFT has demonstrated that its antidepressant efficacy is maintained for periods of up to 44 weeks following 8 weeks of initial treatment at a dose of 50–200 mg/day (mean dose of 70 mg/day) (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Posttraumatic Stress DisorderIt is generally agreed that PTSD requires several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in PTSD is maintained for periods of up to 28 weeks following 24 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Social Anxiety DisorderSocial anxiety disorder is a chronic condition that may require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in social anxiety disorder is maintained for periods of up to 24 weeks following 20 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). Dosage adjustments should be made to maintain patients on the lowest effective dose and patients should be periodically reassessed to determine the need for long-term treatment.
Obsessive-Compulsive Disorder and Panic DisorderIt is generally agreed that OCD and Panic Disorder require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of continuing ZOLOFT for periods of up to 28 weeks in patients with OCD and Panic Disorder who have responded while taking ZOLOFT during initial treatment phases of 24 to 52 weeks of treatment at a dose range of 50–200 mg/day has demonstrated a benefit of such maintenance treatment (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Nevertheless, patients should be periodically reassessed to determine the need for maintenance treatment.
Premenstrual Dysphoric DisorderThe effectiveness of ZOLOFT in long-term use, that is, for more than 3 menstrual cycles, has not been systematically evaluated in controlled trials. However, as women commonly report that symptoms worsen with age until relieved by the onset of menopause, it is reasonable to consider continuation of a responding patient. Dosage adjustments, which may include changes between dosage regimens (e.g., daily throughout the menstrual cycle versus during the luteal phase of the menstrual cycle), may be needed to maintain the patient on the lowest effective dosage and patients should be periodically reassessed to determine the need for continued treatment.
Switching Patients to or from a Monoamine Oxidase InhibitorAt least 14 days should elapse between discontinuation of an MAOI and initiation of therapy with ZOLOFT. In addition, at least 14 days should be allowed after stopping ZOLOFT before starting an MAOI (see CONTRAINDICATIONS and WARNINGS).
Special Populations Dosage for Hepatically Impaired PatientsThe use of sertraline in patients with liver disease should be approached with caution. The effects of sertraline in patients with moderate and severe hepatic impairment have not been studied. If sertraline is administered to patients with liver impairment, a lower or less frequent dose should be used (see CLINICAL PHARMACOLOGY and PRECAUTIONS).
Treatment of Pregnant Women During the Third TrimesterNeonates exposed to ZOLOFT and other SSRIs or SNRIs, late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding (see PRECAUTIONS). When treating pregnant women with ZOLOFT during the third trimester, the physician should carefully consider the potential risks and benefits of treatment. The physician may consider tapering ZOLOFT in the third trimester.
Discontinuation of Treatment with ZoloftSymptoms associated with discontinuation of ZOLOFT and other SSRIs and SNRIs, have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
ZOLOFT Oral ConcentrateZOLOFT Oral Concentrate contains 20 mg/mL of sertraline (as the hydrochloride) as the active ingredient and 12% alcohol. ZOLOFT Oral Concentrate must be diluted before use. Just before taking, use the dropper provided to remove the required amount of ZOLOFT Oral Concentrate and mix with 4 oz (1/2 cup) of water, ginger ale, lemon/lime soda, lemonade or orange juice ONLY. Do not mix ZOLOFT Oral Concentrate with anything other than the liquids listed. The dose should be taken immediately after mixing. Do not mix in advance. At times, a slight haze may appear after mixing; this is normal. Note that caution should be exercised for patients with latex sensitivity, as the dropper dispenser contains dry natural rubber.
ZOLOFT Oral Concentrate is contraindicated with ANTABUSE (disulfiram) due to the alcohol content of the concentrate.
-
Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Zoloft | Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc] Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Initial Treatment Dosage for Adults Major Depressive Disorder and Obsessive-Compulsive DisorderZOLOFT treatment should be administered at a dose of 50 mg once daily.
Panic Disorder, Posttraumatic Stress Disorder and Social Anxiety DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily. After one week, the dose should be increased to 50 mg once daily.
While a relationship between dose and effect has not been established for major depressive disorder, OCD, panic disorder, PTSD or social anxiety disorder, patients were dosed in a range of 50–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for the treatment of these indications. Consequently, a dose of 50 mg, administered once daily, is recommended as the initial therapeutic dose. Patients not responding to a 50 mg dose may benefit from dose increases up to a maximum of 200 mg/day. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
Premenstrual Dysphoric DisorderZOLOFT treatment should be initiated with a dose of 50 mg/day, either daily throughout the menstrual cycle or limited to the luteal phase of the menstrual cycle, depending on physician assessment.
While a relationship between dose and effect has not been established for PMDD, patients were dosed in the range of 50–150 mg/day with dose increases at the onset of each new menstrual cycle (see Clinical Trials under CLINICAL PHARMACOLOGY). Patients not responding to a 50 mg/day dose may benefit from dose increases (at 50 mg increments/menstrual cycle) up to 150 mg/day when dosing daily throughout the menstrual cycle, or 100 mg/day when dosing during the luteal phase of the menstrual cycle. If a 100 mg/day dose has been established with luteal phase dosing, a 50 mg/day titration step for three days should be utilized at the beginning of each luteal phase dosing period.
ZOLOFT should be administered once daily, either in the morning or evening.
Dosage for Pediatric Population (Children and Adolescents) Obsessive-Compulsive DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily in children (ages 6–12) and at a dose of 50 mg once daily in adolescents (ages 13–17).
While a relationship between dose and effect has not been established for OCD, patients were dosed in a range of 25–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for pediatric patients (6–17 years) with OCD. Patients not responding to an initial dose of 25 or 50 mg/day may benefit from dose increases up to a maximum of 200 mg/day. For children with OCD, their generally lower body weights compared to adults should be taken into consideration in advancing the dose, in order to avoid excess dosing. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
ZOLOFT should be administered once daily, either in the morning or evening.
Maintenance/Continuation/Extended Treatment Major Depressive DisorderIt is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy beyond response to the acute episode. Systematic evaluation of ZOLOFT has demonstrated that its antidepressant efficacy is maintained for periods of up to 44 weeks following 8 weeks of initial treatment at a dose of 50–200 mg/day (mean dose of 70 mg/day) (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Posttraumatic Stress DisorderIt is generally agreed that PTSD requires several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in PTSD is maintained for periods of up to 28 weeks following 24 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Social Anxiety DisorderSocial anxiety disorder is a chronic condition that may require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in social anxiety disorder is maintained for periods of up to 24 weeks following 20 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). Dosage adjustments should be made to maintain patients on the lowest effective dose and patients should be periodically reassessed to determine the need for long-term treatment.
Obsessive-Compulsive Disorder and Panic DisorderIt is generally agreed that OCD and Panic Disorder require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of continuing ZOLOFT for periods of up to 28 weeks in patients with OCD and Panic Disorder who have responded while taking ZOLOFT during initial treatment phases of 24 to 52 weeks of treatment at a dose range of 50–200 mg/day has demonstrated a benefit of such maintenance treatment (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Nevertheless, patients should be periodically reassessed to determine the need for maintenance treatment.
Premenstrual Dysphoric DisorderThe effectiveness of ZOLOFT in long-term use, that is, for more than 3 menstrual cycles, has not been systematically evaluated in controlled trials. However, as women commonly report that symptoms worsen with age until relieved by the onset of menopause, it is reasonable to consider continuation of a responding patient. Dosage adjustments, which may include changes between dosage regimens (e.g., daily throughout the menstrual cycle versus during the luteal phase of the menstrual cycle), may be needed to maintain the patient on the lowest effective dosage and patients should be periodically reassessed to determine the need for continued treatment.
Switching Patients to or from a Monoamine Oxidase InhibitorAt least 14 days should elapse between discontinuation of an MAOI and initiation of therapy with ZOLOFT. In addition, at least 14 days should be allowed after stopping ZOLOFT before starting an MAOI (see CONTRAINDICATIONS and WARNINGS).
Special Populations Dosage for Hepatically Impaired PatientsThe use of sertraline in patients with liver disease should be approached with caution. The effects of sertraline in patients with moderate and severe hepatic impairment have not been studied. If sertraline is administered to patients with liver impairment, a lower or less frequent dose should be used (see CLINICAL PHARMACOLOGY and PRECAUTIONS).
Treatment of Pregnant Women During the Third TrimesterNeonates exposed to ZOLOFT and other SSRIs or SNRIs, late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding (see PRECAUTIONS). When treating pregnant women with ZOLOFT during the third trimester, the physician should carefully consider the potential risks and benefits of treatment. The physician may consider tapering ZOLOFT in the third trimester.
Discontinuation of Treatment with ZoloftSymptoms associated with discontinuation of ZOLOFT and other SSRIs and SNRIs, have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
ZOLOFT Oral ConcentrateZOLOFT Oral Concentrate contains 20 mg/mL of sertraline (as the hydrochloride) as the active ingredient and 12% alcohol. ZOLOFT Oral Concentrate must be diluted before use. Just before taking, use the dropper provided to remove the required amount of ZOLOFT Oral Concentrate and mix with 4 oz (1/2 cup) of water, ginger ale, lemon/lime soda, lemonade or orange juice ONLY. Do not mix ZOLOFT Oral Concentrate with anything other than the liquids listed. The dose should be taken immediately after mixing. Do not mix in advance. At times, a slight haze may appear after mixing; this is normal. Note that caution should be exercised for patients with latex sensitivity, as the dropper dispenser contains dry natural rubber.
ZOLOFT Oral Concentrate is contraindicated with ANTABUSE (disulfiram) due to the alcohol content of the concentrate.
-
Cardinal Health
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Cardinal Health]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Zoloft | Cardinal Health
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Cardinal Health] Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Cardinal Health]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Initial Treatment Dosage for Adults Major Depressive Disorder and Obsessive-Compulsive DisorderZOLOFT treatment should be administered at a dose of 50 mg once daily.
Panic Disorder, Posttraumatic Stress Disorder and Social Anxiety DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily. After one week, the dose should be increased to 50 mg once daily.
While a relationship between dose and effect has not been established for major depressive disorder, OCD, panic disorder, PTSD or social anxiety disorder, patients were dosed in a range of 50–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for the treatment of these indications. Consequently, a dose of 50 mg, administered once daily, is recommended as the initial therapeutic dose. Patients not responding to a 50 mg dose may benefit from dose increases up to a maximum of 200 mg/day. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
Premenstrual Dysphoric DisorderZOLOFT treatment should be initiated with a dose of 50 mg/day, either daily throughout the menstrual cycle or limited to the luteal phase of the menstrual cycle, depending on physician assessment.
While a relationship between dose and effect has not been established for PMDD, patients were dosed in the range of 50–150 mg/day with dose increases at the onset of each new menstrual cycle (see Clinical Trials under CLINICAL PHARMACOLOGY). Patients not responding to a 50 mg/day dose may benefit from dose increases (at 50 mg increments/menstrual cycle) up to 150 mg/day when dosing daily throughout the menstrual cycle, or 100 mg/day when dosing during the luteal phase of the menstrual cycle. If a 100 mg/day dose has been established with luteal phase dosing, a 50 mg/day titration step for three days should be utilized at the beginning of each luteal phase dosing period.
ZOLOFT should be administered once daily, either in the morning or evening.
Dosage for Pediatric Population (Children and Adolescents) Obsessive-Compulsive DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily in children (ages 6–12) and at a dose of 50 mg once daily in adolescents (ages 13–17).
While a relationship between dose and effect has not been established for OCD, patients were dosed in a range of 25–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for pediatric patients (6–17 years) with OCD. Patients not responding to an initial dose of 25 or 50 mg/day may benefit from dose increases up to a maximum of 200 mg/day. For children with OCD, their generally lower body weights compared to adults should be taken into consideration in advancing the dose, in order to avoid excess dosing. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
ZOLOFT should be administered once daily, either in the morning or evening.
Maintenance/Continuation/Extended Treatment Major Depressive DisorderIt is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy beyond response to the acute episode. Systematic evaluation of ZOLOFT has demonstrated that its antidepressant efficacy is maintained for periods of up to 44 weeks following 8 weeks of initial treatment at a dose of 50–200 mg/day (mean dose of 70 mg/day) (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Posttraumatic Stress DisorderIt is generally agreed that PTSD requires several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in PTSD is maintained for periods of up to 28 weeks following 24 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Social Anxiety DisorderSocial anxiety disorder is a chronic condition that may require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in social anxiety disorder is maintained for periods of up to 24 weeks following 20 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). Dosage adjustments should be made to maintain patients on the lowest effective dose and patients should be periodically reassessed to determine the need for long-term treatment.
Obsessive-Compulsive Disorder and Panic DisorderIt is generally agreed that OCD and Panic Disorder require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of continuing ZOLOFT for periods of up to 28 weeks in patients with OCD and Panic Disorder who have responded while taking ZOLOFT during initial treatment phases of 24 to 52 weeks of treatment at a dose range of 50–200 mg/day has demonstrated a benefit of such maintenance treatment (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Nevertheless, patients should be periodically reassessed to determine the need for maintenance treatment.
Premenstrual Dysphoric DisorderThe effectiveness of ZOLOFT in long-term use, that is, for more than 3 menstrual cycles, has not been systematically evaluated in controlled trials. However, as women commonly report that symptoms worsen with age until relieved by the onset of menopause, it is reasonable to consider continuation of a responding patient. Dosage adjustments, which may include changes between dosage regimens (e.g., daily throughout the menstrual cycle versus during the luteal phase of the menstrual cycle), may be needed to maintain the patient on the lowest effective dosage and patients should be periodically reassessed to determine the need for continued treatment.
Switching Patients to or from a Monoamine Oxidase InhibitorAt least 14 days should elapse between discontinuation of an MAOI and initiation of therapy with ZOLOFT. In addition, at least 14 days should be allowed after stopping ZOLOFT before starting an MAOI (see CONTRAINDICATIONS and WARNINGS).
Special Populations Dosage for Hepatically Impaired PatientsThe use of sertraline in patients with liver disease should be approached with caution. The effects of sertraline in patients with moderate and severe hepatic impairment have not been studied. If sertraline is administered to patients with liver impairment, a lower or less frequent dose should be used (see CLINICAL PHARMACOLOGY and PRECAUTIONS).
Treatment of Pregnant Women During the Third TrimesterNeonates exposed to ZOLOFT and other SSRIs or SNRIs, late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding (see PRECAUTIONS). When treating pregnant women with ZOLOFT during the third trimester, the physician should carefully consider the potential risks and benefits of treatment. The physician may consider tapering ZOLOFT in the third trimester.
Discontinuation of Treatment with ZoloftSymptoms associated with discontinuation of ZOLOFT and other SSRIs and SNRIs, have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
ZOLOFT Oral ConcentrateZOLOFT Oral Concentrate contains 20 mg/mL of sertraline (as the hydrochloride) as the active ingredient and 12% alcohol. ZOLOFT Oral Concentrate must be diluted before use. Just before taking, use the dropper provided to remove the required amount of ZOLOFT Oral Concentrate and mix with 4 oz (1/2 cup) of water, ginger ale, lemon/lime soda, lemonade or orange juice ONLY. Do not mix ZOLOFT Oral Concentrate with anything other than the liquids listed. The dose should be taken immediately after mixing. Do not mix in advance. At times, a slight haze may appear after mixing; this is normal. Note that caution should be exercised for patients with latex sensitivity, as the dropper dispenser contains dry natural rubber.
ZOLOFT Oral Concentrate is contraindicated with ANTABUSE (disulfiram) due to the alcohol content of the concentrate.
-
Remedyrepack Inc.
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Remedyrepack Inc. ]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Zoloft | Remedyrepack Inc.
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Remedyrepack Inc. ] Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Remedyrepack Inc. ]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
ZOLOFT treatment should be administered at a dose of 50 mg once daily.
ZOLOFT treatment should be initiated with a dose of 25 mg once daily. After one week, the dose should be increased to 50 mg once daily.
While a relationship between dose and effect has not been established for major depressive disorder, OCD, panic disorder, PTSD or social anxiety disorder, patients were dosed in a range of 50–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for the treatment of these indications. Consequently, a dose of 50 mg, administered once daily, is recommended as the initial therapeutic dose. Patients not responding to a 50 mg dose may benefit from dose increases up to a maximum of 200 mg/day. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
ZOLOFT treatment should be initiated with a dose of 50 mg/day, either daily throughout the menstrual cycle or limited to the luteal phase of the menstrual cycle, depending on physician assessment.
While a relationship between dose and effect has not been established for PMDD, patients were dosed in the range of 50–150 mg/day with dose increases at the onset of each new menstrual cycle (see Clinical Trials under CLINICAL PHARMACOLOGY). Patients not responding to a 50 mg/day dose may benefit from dose increases (at 50 mg increments/menstrual cycle) up to 150 mg/day when dosing daily throughout the menstrual cycle, or 100 mg/day when dosing during the luteal phase of the menstrual cycle. If a 100 mg/day dose has been established with luteal phase dosing, a 50 mg/day titration step for three days should be utilized at the beginning of each luteal phase dosing period.
ZOLOFT should be administered once daily, either in the morning or evening.
ZOLOFT treatment should be initiated with a dose of 25 mg once daily in children (ages 6–12) and at a dose of 50 mg once daily in adolescents (ages 13–17).
While a relationship between dose and effect has not been established for OCD, patients were dosed in a range of 25–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for pediatric patients (6–17 years) with OCD. Patients not responding to an initial dose of 25 or 50 mg/day may benefit from dose increases up to a maximum of 200 mg/day. For children with OCD, their generally lower body weights compared to adults should be taken into consideration in advancing the dose, in order to avoid excess dosing. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
ZOLOFT should be administered once daily, either in the morning or evening.
It is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy beyond response to the acute episode. Systematic evaluation of ZOLOFT has demonstrated that its antidepressant efficacy is maintained for periods of up to 44 weeks following 8 weeks of initial treatment at a dose of 50–200 mg/day (mean dose of 70 mg/day) (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
It is generally agreed that PTSD requires several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in PTSD is maintained for periods of up to 28 weeks following 24 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Social anxiety disorder is a chronic condition that may require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in social anxiety disorder is maintained for periods of up to 24 weeks following 20 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). Dosage adjustments should be made to maintain patients on the lowest effective dose and patients should be periodically reassessed to determine the need for long-term treatment.
It is generally agreed that OCD and Panic Disorder require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of continuing ZOLOFT for periods of up to 28 weeks in patients with OCD and Panic Disorder who have responded while taking ZOLOFT during initial treatment phases of 24 to 52 weeks of treatment at a dose range of 50–200 mg/day has demonstrated a benefit of such maintenance treatment (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Nevertheless, patients should be periodically reassessed to determine the need for maintenance treatment.
The effectiveness of ZOLOFT in long-term use, that is, for more than 3 menstrual cycles, has not been systematically evaluated in controlled trials. However, as women commonly report that symptoms worsen with age until relieved by the onset of menopause, it is reasonable to consider continuation of a responding patient. Dosage adjustments, which may include changes between dosage regimens (e.g., daily throughout the menstrual cycle versus during the luteal phase of the menstrual cycle), may be needed to maintain the patient on the lowest effective dosage and patients should be periodically reassessed to determine the need for continued treatment.
At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with ZOLOFT. Conversely, at least 14 days should be allowed after stopping ZOLOFT before starting an MAOI intended to treat psychiatric disorders (see CONTRAINDICATIONS).
Do not start ZOLOFT in a patient who is being treated with linezolid or intravenous methylene blue because there is increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered (see CONTRAINDICATIONS).
In some cases, a patient already receiving ZOLOFT therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, ZOLOFT should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with ZOLOFT may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue (see WARNINGS).
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with ZOLOFT is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use (see WARNINGS).
The use of sertraline in patients with liver disease should be approached with caution. The effects of sertraline in patients with moderate and severe hepatic impairment have not been studied. If sertraline is administered to patients with liver impairment, a lower or less frequent dose should be used (see CLINICAL PHARMACOLOGY and PRECAUTIONS).
Neonates exposed to ZOLOFT and other SSRIs or SNRIs, late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding (see PRECAUTIONS). When treating pregnant women with ZOLOFT during the third trimester, the physician should carefully consider the potential risks and benefits of treatment.
Symptoms associated with discontinuation of ZOLOFT and other SSRIs and SNRIs, have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
ZOLOFT Oral Concentrate contains 20 mg/mL of sertraline (as the hydrochloride) as the active ingredient and 12% alcohol. ZOLOFT Oral Concentrate must be diluted before use. Just before taking, use the dropper provided to remove the required amount of ZOLOFT Oral Concentrate and mix with 4 oz (1/2 cup) of water, ginger ale, lemon/lime soda, lemonade or orange juice ONLY. Do not mix ZOLOFT Oral Concentrate with anything other than the liquids listed. The dose should be taken immediately after mixing. Do not mix in advance. At times, a slight haze may appear after mixing; this is normal. Note that caution should be exercised for patients with latex sensitivity, as the dropper dispenser contains dry natural rubber.
ZOLOFT Oral Concentrate is contraindicated with ANTABUSE (disulfiram) due to the alcohol content of the concentrate.
-
Aphena Pharma Solutions – Tennessee, Llc
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Aphena Pharma Solutions – Tennessee, Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Zoloft | Aphena Pharma Solutions - Tennessee, Llc
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Aphena Pharma Solutions – Tennessee, Llc] Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Aphena Pharma Solutions – Tennessee, Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Initial Treatment Dosage for Adults Major Depressive Disorder and Obsessive-Compulsive DisorderZOLOFT treatment should be administered at a dose of 50 mg once daily.
Panic Disorder, Posttraumatic Stress Disorder and Social Anxiety DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily. After one week, the dose should be increased to 50 mg once daily.
While a relationship between dose and effect has not been established for major depressive disorder, OCD, panic disorder, PTSD or social anxiety disorder, patients were dosed in a range of 50–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for the treatment of these indications. Consequently, a dose of 50 mg, administered once daily, is recommended as the initial therapeutic dose. Patients not responding to a 50 mg dose may benefit from dose increases up to a maximum of 200 mg/day. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
Premenstrual Dysphoric DisorderZOLOFT treatment should be initiated with a dose of 50 mg/day, either daily throughout the menstrual cycle or limited to the luteal phase of the menstrual cycle, depending on physician assessment.
While a relationship between dose and effect has not been established for PMDD, patients were dosed in the range of 50–150 mg/day with dose increases at the onset of each new menstrual cycle (see Clinical Trials under CLINICAL PHARMACOLOGY). Patients not responding to a 50 mg/day dose may benefit from dose increases (at 50 mg increments/menstrual cycle) up to 150 mg/day when dosing daily throughout the menstrual cycle, or 100 mg/day when dosing during the luteal phase of the menstrual cycle. If a 100 mg/day dose has been established with luteal phase dosing, a 50 mg/day titration step for three days should be utilized at the beginning of each luteal phase dosing period.
ZOLOFT should be administered once daily, either in the morning or evening.
Dosage for Pediatric Population (Children and Adolescents) Obsessive-Compulsive DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily in children (ages 6–12) and at a dose of 50 mg once daily in adolescents (ages 13–17).
While a relationship between dose and effect has not been established for OCD, patients were dosed in a range of 25–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for pediatric patients (6–17 years) with OCD. Patients not responding to an initial dose of 25 or 50 mg/day may benefit from dose increases up to a maximum of 200 mg/day. For children with OCD, their generally lower body weights compared to adults should be taken into consideration in advancing the dose, in order to avoid excess dosing. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
ZOLOFT should be administered once daily, either in the morning or evening.
Maintenance/Continuation/Extended Treatment Major Depressive DisorderIt is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy beyond response to the acute episode. Systematic evaluation of ZOLOFT has demonstrated that its antidepressant efficacy is maintained for periods of up to 44 weeks following 8 weeks of initial treatment at a dose of 50–200 mg/day (mean dose of 70 mg/day) (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Posttraumatic Stress DisorderIt is generally agreed that PTSD requires several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in PTSD is maintained for periods of up to 28 weeks following 24 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Social Anxiety DisorderSocial anxiety disorder is a chronic condition that may require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in social anxiety disorder is maintained for periods of up to 24 weeks following 20 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). Dosage adjustments should be made to maintain patients on the lowest effective dose and patients should be periodically reassessed to determine the need for long-term treatment.
Obsessive-Compulsive Disorder and Panic DisorderIt is generally agreed that OCD and Panic Disorder require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of continuing ZOLOFT for periods of up to 28 weeks in patients with OCD and Panic Disorder who have responded while taking ZOLOFT during initial treatment phases of 24 to 52 weeks of treatment at a dose range of 50–200 mg/day has demonstrated a benefit of such maintenance treatment (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Nevertheless, patients should be periodically reassessed to determine the need for maintenance treatment.
Premenstrual Dysphoric DisorderThe effectiveness of ZOLOFT in long-term use, that is, for more than 3 menstrual cycles, has not been systematically evaluated in controlled trials. However, as women commonly report that symptoms worsen with age until relieved by the onset of menopause, it is reasonable to consider continuation of a responding patient. Dosage adjustments, which may include changes between dosage regimens (e.g., daily throughout the menstrual cycle versus during the luteal phase of the menstrual cycle), may be needed to maintain the patient on the lowest effective dosage and patients should be periodically reassessed to determine the need for continued treatment.
Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric DisordersAt least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with ZOLOFT. Conversely, at least 14 days should be allowed after stopping ZOLOFT before starting an MAOI intended to treat psychiatric disorders (see CONTRAINDICATIONS).
Use of ZOLOFT With Other MAOIs Such as Linezolid or Methylene BlueDo not start ZOLOFT in a patient who is being treated with linezolid or intravenous methylene blue because there is increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered (see CONTRAINDICATIONS).
In some cases, a patient already receiving ZOLOFT therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, ZOLOFT should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with ZOLOFT may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue (see WARNINGS).
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with ZOLOFT is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use (see WARNINGS).
Special Populations Dosage for Hepatically Impaired PatientsThe use of sertraline in patients with liver disease should be approached with caution. The effects of sertraline in patients with moderate and severe hepatic impairment have not been studied. If sertraline is administered to patients with liver impairment, a lower or less frequent dose should be used (see CLINICAL PHARMACOLOGY and PRECAUTIONS).
Treatment of Pregnant Women During the Third TrimesterNeonates exposed to ZOLOFT and other SSRIs or SNRIs, late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding (see PRECAUTIONS). When treating pregnant women with ZOLOFT during the third trimester, the physician should carefully consider the potential risks and benefits of treatment.
Discontinuation of Treatment with ZOLOFTSymptoms associated with discontinuation of ZOLOFT and other SSRIs and SNRIs, have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
ZOLOFT Oral ConcentrateZOLOFT Oral Concentrate contains 20 mg/mL of sertraline (as the hydrochloride) as the active ingredient and 12% alcohol. ZOLOFT Oral Concentrate must be diluted before use. Just before taking, use the dropper provided to remove the required amount of ZOLOFT Oral Concentrate and mix with 4 oz (1/2 cup) of water, ginger ale, lemon/lime soda, lemonade or orange juice ONLY. Do not mix ZOLOFT Oral Concentrate with anything other than the liquids listed. The dose should be taken immediately after mixing. Do not mix in advance. At times, a slight haze may appear after mixing; this is normal. Note that caution should be exercised for patients with latex sensitivity, as the dropper dispenser contains dry natural rubber.
ZOLOFT Oral Concentrate is contraindicated with ANTABUSE (disulfiram) due to the alcohol content of the concentrate.
-
Roerig
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated Zoloft (Sertraline Hydrochloride) Solution, Concentrate [Roerig]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Zoloft | Roerig
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated Zoloft (Sertraline Hydrochloride) Solution, Concentrate [Roerig] Zoloft (Sertraline Hydrochloride) Tablet, Film Coated Zoloft (Sertraline Hydrochloride) Solution, Concentrate [Roerig]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Initial Treatment Dosage for Adults Major Depressive Disorder and Obsessive-Compulsive DisorderZOLOFT treatment should be administered at a dose of 50 mg once daily.
Panic Disorder, Posttraumatic Stress Disorder and Social Anxiety DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily. After one week, the dose should be increased to 50 mg once daily.
While a relationship between dose and effect has not been established for major depressive disorder, OCD, panic disorder, PTSD or social anxiety disorder, patients were dosed in a range of 50–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for the treatment of these indications. Consequently, a dose of 50 mg, administered once daily, is recommended as the initial therapeutic dose. Patients not responding to a 50 mg dose may benefit from dose increases up to a maximum of 200 mg/day. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
Premenstrual Dysphoric DisorderZOLOFT treatment should be initiated with a dose of 50 mg/day, either daily throughout the menstrual cycle or limited to the luteal phase of the menstrual cycle, depending on physician assessment.
While a relationship between dose and effect has not been established for PMDD, patients were dosed in the range of 50–150 mg/day with dose increases at the onset of each new menstrual cycle (see Clinical Trials under CLINICAL PHARMACOLOGY). Patients not responding to a 50 mg/day dose may benefit from dose increases (at 50 mg increments/menstrual cycle) up to 150 mg/day when dosing daily throughout the menstrual cycle, or 100 mg/day when dosing during the luteal phase of the menstrual cycle. If a 100 mg/day dose has been established with luteal phase dosing, a 50 mg/day titration step for three days should be utilized at the beginning of each luteal phase dosing period.
ZOLOFT should be administered once daily, either in the morning or evening.
Dosage for Pediatric Population (Children and Adolescents) Obsessive-Compulsive DisorderZOLOFT treatment should be initiated with a dose of 25 mg once daily in children (ages 6–12) and at a dose of 50 mg once daily in adolescents (ages 13–17).
While a relationship between dose and effect has not been established for OCD, patients were dosed in a range of 25–200 mg/day in the clinical trials demonstrating the effectiveness of ZOLOFT for pediatric patients (6–17 years) with OCD. Patients not responding to an initial dose of 25 or 50 mg/day may benefit from dose increases up to a maximum of 200 mg/day. For children with OCD, their generally lower body weights compared to adults should be taken into consideration in advancing the dose, in order to avoid excess dosing. Given the 24 hour elimination half-life of ZOLOFT, dose changes should not occur at intervals of less than 1 week.
ZOLOFT should be administered once daily, either in the morning or evening.
Maintenance/Continuation/Extended Treatment Major Depressive DisorderIt is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy beyond response to the acute episode. Systematic evaluation of ZOLOFT has demonstrated that its antidepressant efficacy is maintained for periods of up to 44 weeks following 8 weeks of initial treatment at a dose of 50–200 mg/day (mean dose of 70 mg/day) (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Posttraumatic Stress DisorderIt is generally agreed that PTSD requires several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in PTSD is maintained for periods of up to 28 weeks following 24 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment.
Social Anxiety DisorderSocial anxiety disorder is a chronic condition that may require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of ZOLOFT has demonstrated that its efficacy in social anxiety disorder is maintained for periods of up to 24 weeks following 20 weeks of treatment at a dose of 50–200 mg/day (see Clinical Trials under CLINICAL PHARMACOLOGY). Dosage adjustments should be made to maintain patients on the lowest effective dose and patients should be periodically reassessed to determine the need for long-term treatment.
Obsessive-Compulsive Disorder and Panic DisorderIt is generally agreed that OCD and Panic Disorder require several months or longer of sustained pharmacological therapy beyond response to initial treatment. Systematic evaluation of continuing ZOLOFT for periods of up to 28 weeks in patients with OCD and Panic Disorder who have responded while taking ZOLOFT during initial treatment phases of 24 to 52 weeks of treatment at a dose range of 50–200 mg/day has demonstrated a benefit of such maintenance treatment (see Clinical Trials under CLINICAL PHARMACOLOGY). It is not known whether the dose of ZOLOFT needed for maintenance treatment is identical to the dose needed to achieve an initial response. Nevertheless, patients should be periodically reassessed to determine the need for maintenance treatment.
Premenstrual Dysphoric DisorderThe effectiveness of ZOLOFT in long-term use, that is, for more than 3 menstrual cycles, has not been systematically evaluated in controlled trials. However, as women commonly report that symptoms worsen with age until relieved by the onset of menopause, it is reasonable to consider continuation of a responding patient. Dosage adjustments, which may include changes between dosage regimens (e.g., daily throughout the menstrual cycle versus during the luteal phase of the menstrual cycle), may be needed to maintain the patient on the lowest effective dosage and patients should be periodically reassessed to determine the need for continued treatment.
Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric DisordersAt least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with ZOLOFT. Conversely, at least 14 days should be allowed after stopping ZOLOFT before starting an MAOI intended to treat psychiatric disorders (see CONTRAINDICATIONS).
Use of ZOLOFT With Other MAOIs Such as Linezolid or Methylene BlueDo not start ZOLOFT in a patient who is being treated with linezolid or intravenous methylene blue because there is increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered (see CONTRAINDICATIONS).
In some cases, a patient already receiving ZOLOFT therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, ZOLOFT should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with ZOLOFT may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue (see WARNINGS).
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with ZOLOFT is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use (see WARNINGS).
Special Populations Dosage for Hepatically Impaired PatientsThe use of sertraline in patients with liver disease should be approached with caution. The effects of sertraline in patients with moderate and severe hepatic impairment have not been studied. If sertraline is administered to patients with liver impairment, a lower or less frequent dose should be used (see CLINICAL PHARMACOLOGY and PRECAUTIONS).
Treatment of Pregnant Women During the Third TrimesterNeonates exposed to ZOLOFT and other SSRIs or SNRIs, late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding (see PRECAUTIONS). When treating pregnant women with ZOLOFT during the third trimester, the physician should carefully consider the potential risks and benefits of treatment.
Discontinuation of Treatment with ZOLOFTSymptoms associated with discontinuation of ZOLOFT and other SSRIs and SNRIs, have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
ZOLOFT Oral ConcentrateZOLOFT Oral Concentrate contains 20 mg/mL of sertraline (as the hydrochloride) as the active ingredient and 12% alcohol. ZOLOFT Oral Concentrate must be diluted before use. Just before taking, use the dropper provided to remove the required amount of ZOLOFT Oral Concentrate and mix with 4 oz (1/2 cup) of water, ginger ale, lemon/lime soda, lemonade or orange juice ONLY. Do not mix ZOLOFT Oral Concentrate with anything other than the liquids listed. The dose should be taken immediately after mixing. Do not mix in advance. At times, a slight haze may appear after mixing; this is normal. Note that caution should be exercised for patients with latex sensitivity, as the dropper dispenser contains dry natural rubber.
ZOLOFT Oral Concentrate is contraindicated with ANTABUSE (disulfiram) due to the alcohol content of the concentrate.
Login To Your Free Account

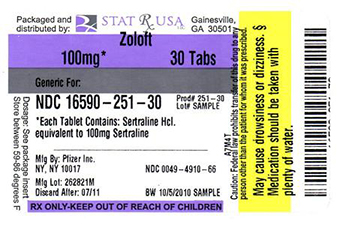



![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Stat Rx Usa Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=c3b0b1f8-abfd-4054-8645-fd076305f4f8&name=ZOLOFT_100MG_LABEL_251.jpg)
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Pd-rx Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=2d52e980-c419-4719-b928-29ad1f71647e&name=55289409.jpg)
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1611926a-6875-4c48-8cb5-54fca7871ced&name=Zoloft100mgRoerig.jpg)
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Cardinal Health]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=162ba178-a30a-49f2-b7a3-4147f4e4dab8&name=162ba178-a30a-49f2-b7a3-4147f4e4dab8-03.jpg)
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=34d6090d-f833-4890-b3b9-c7a9c2fc4630&name=MM4.jpg)
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated [Aphena Pharma Solutions – Tennessee, Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=92278f12-0f00-4c0b-bb44-c6dd2dbb5b6c&name=67544-080.jpg)
![Zoloft (Sertraline Hydrochloride) Tablet, Film Coated Zoloft (Sertraline Hydrochloride) Solution, Concentrate [Roerig]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=fe9e8b7d-61ea-409d-84aa-3ebd79a046b5&name=zoloft-03.jpg)